ORGANIC REACTIONS AND THEIR MECHANISMS
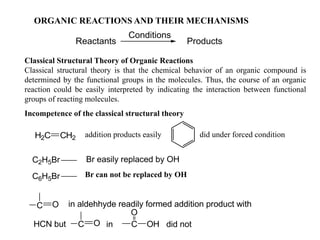
- 1. ORGANIC REACTIONS AND THEIR MECHANISMS Reactants Conditions Products Classical Structural Theory of Organic Reactions Classical structural theory is that the chemical behavior of an organic compound is determined by the functional groups in the molecules. Thus, the course of an organic reaction could be easily interpreted by indicating the interaction between functional groups of reacting molecules. Incompetence of the classical structural theory H2C CH2 C2H5Br Br easily replaced by OH C6H5Br C O in aldehhyde readily formed addition product with in C O OH did notC OHCN but addition products easily did under forced condition Br can not be replaced by OH
- 2. Reaction Mechanism -- Fundamental Aspects Substrate: A substrate may be defined as the reactant that contain carbon atoms some of whose bonds with other atoms are broken and some new bonds are formed as a result of reaction with an attacking agent. The carbon bonds in the substrate molecule are broken or cleaved to give fragments which are very reactive and constitute transitory intermediate. At once they may react with other similar species or with molecules in their environment, thus establishing the new bonds to give product. Attacking agents: For a mechanistic approach, an organic reaction is believed to take place by the attack of a reagent on a compound containing carbon (substrate) and the reagent is known as attacking agents. Attacking reagent bear either +ve or -ve charge. Therefore, attacking agent will not attack the substrate successfully unless the latter somehow possessed oppositely charged center in the mole. Substrate molecule must develop polarity on some of its carbon atoms and constituents linked together. Polarity is developed by the displacement of bonded electrons. Attacking Reagent + Substrate Product Substrate Product (Transitory) Intermediate Attacking Reagent bear either +ve or -ve charge Substrate ------- electrically neutral ------- develop polarity on some of its carbon atom
- 3. Bond Fission: Bond fission means separation of two atoms from each other in a covalently bonded molecule. Bond Fission Homolytic fission Heterolytic fission Each of the atoms acquire one of the bonded electrons One of the atoms acquire both of the bonded electrons because A is more electronegative than B which thereby acquires both the bonding electrons and becomes negatively charged. A B A + Heterolytic fissionB - + A B A + Homolytic fissionB Light Energy
- 4. Carbonium Ions: An organic ion containing a positively charged carbon atom is called a carbonium ion C H H H C H H H X Heterolytic fission + X - + C H H H C H H3C H C CH3 H3C H C CH3 CH3 H3C 10 20 30 + + + + C R R R + C H H R +C R H R +> > 30 20 10
- 5. THE RELATIVE STABILITIES OF CARBOCATIONS Carbanium ion being deficient in electrons is very unstable. Electron realising group such as alkyl group adjacent to the carbon atom makes the carbanium ion stable by partial neutraliztion of the positive charge on carbon Alkyl groups, when compared to hydrogen atoms, are electron releasing thus the order of stability of carbocations parallel the number of attached alkyl / methyl groups. C R R R + C H H R +C R H R +> > 30 20 10 C H H H + > The relative stabilities of carbocations is 3° > 2° > 1° > methyl
- 6. Molecular rearrangement /Carbocation Rearrangements When carbocations are intermediates, a less stable carbocation can rearrange to a more stable carbocation by a shift of a hydrogen or an alkyl group. This is called a carbocation rearrangements. The migrating group in a 1,2-shift moves with two bonding electrons, giving carbon a net positive (+) charge. A 1,2-shift converts a less stable carbocation to a more stable carbocation. C C H C C CC HH + C C R C C CC RR + 1,2 Hydride shift: Movement of a hydrogen atom is called 1,2 Hydride shift 1,2 alkyl shift: Movement of an alkyl group is called 1,2 alkyl shift
- 7. CH3 C C CH3 CH3 CH3 H CH3 C C CH3 CH3 H CH3 2° carbocation 3° carbocation 1,2 methyl shift H CH3 C H C H H CH3 C H C H H H 1,2 Hydride shift 1° carbocation 2° carbocation
- 8. Reactions of Carbonium ion Proton loss CH3 CH CH2 H - H+ CH3 CH CH2 Propylene + Combination with nucleophile Addition to an alkene Abstraction of Hydride ion CH3 C +CH2 CH3 CH3 C CH3 CH3 CH3 C CH2 CH3 CH3 C CH3 CH3 CH3 CCH2 CH3CH3 C CH3 H3C CH3 C CH3 CH3H+ CH3 CHCH2 CH3CH3 C CH3 H3C CH3 C CH3 CH3+ CH3 CH2 CH3 CH2 Br+ Br
- 9. Carbanions: An organic ion with a pair of electrons and a negative charge on the central carbon atom is called a carbanion (carb, from carbon + anion, negative charge) C H H H C H H H Y Heterolytic fission + Y - + H C CH2 O - C O+ H C CH2 O C O - Reactions of Carbanion Addition reaction Substitution reaction Na+ HC OOCC2H5 OOCC2H5 CH3 I - + HC OOCC2H5 OOCC2H5 NaI+CH3
- 10. Stability of Carbanions: Carbanions being rich in electrons is very unstable, so electron attracting/withdrawing group such as CN, NO2 group makes the carbanion more stable +- C CN CH > - - More stable On the other hand, electron realising group such as alkyl group adjacent to the carbon atom makes the carbanion ion less stable by increasing the electron density on the negatively charged carbon. The relative stabilities of carbanion is Methyl > 1° > 2° > 3° C H H H C H H3C H C CH3 H3C H C CH3 CH3 H3C 10 20 30 > > >
- 11. Homolytic bond fission means separation of two atoms in which each of the atoms acquire one of the bonded electrons A B A + Homolytic fissionB Light Energy Free radicals: An atom or group of atoms possessing an odd (unpaired) electrons is known as free radical. A dot generally represents the odd electrons, the symbol of free radical. They are produced by homolytic fission of the molecule A : B A + B. .Light/heat
- 12. Alkyl radicals are classified as being 1°, 2°, or 3° on the basis of the carbon atom that has the unpaired electron. Propyl radical (a 1° radical) Isopropyl radical (a 2° radical) The relative stabilities of alkyl radicals: The order of stability of alkyl radicals is the same as for carbocations. Alkyl radicals are uncharged, the carbon that bears the odd electrons is electron deficient. Therefore, electron-releasing alkyl groups attached to this carbon provide a stabilizing effect, and more alkyl groups that are attached to this carbon, the more stable the radical is.
- 13. Electrophiles: An agent which can accept an electron pair in a reaction is called electrophile. Electrophiles are electron-deficient. The name electrophile means (electro = electrons and philic = loving) “electron-loving” and indicates that it attacts regions of high electron density (negative centre) of the substrate molecules. Electrophiles Positive electrophiles Neutral electrophiles They are deficient of two electrons and carry a positive charge Neutral molecules with electron deficient centre C+ H H H Br :: : + B F F F Al Cl Cl Cl Nucleophile: An agent which can donate an electron pair in a reaction is called nucleophile. Nucleophiles are electron rich. The name nucleophile means (nucleo = nucleus and philic = loving) “nucleus-loving”. Since the nucleus is electrically positive, the nucleophile will attacts regions of low electron density (positive centre) of the substrate molecules Nucleophiles Negative Nucleophiles Neutral Nucleophiles C: H H H - An excess electron pair and carry a negative charge N: H H H Br :: :: Posses spare electron pair and no charge
- 14. Electron Displacement Effects Inductive effect: The permanent effect whereby polarity is induced on the carbon atom and the substituent attached to it due to minor displacement of bonding electron pair caused by their different electronegativities, is known as inductive effect or simply as I-effect. Inductive effect (I effect) refers to polarity produced in a molecule as a result of higher electronegativity of one atom compared to other Inductive effect Positive Inductive Effect (+ I effect) Negative Inductive Effect (- I effect) C X C : X C X C Y C : Y C Y The substituent bonded to carbon atom is electron attracting /withdrawing group, it develops negative charge and the inductive effect is called negative inductive effect (-I effect) The substituent bonded to carbon atom which loses electrons toward carbon atom (electron releasing group), it develops positive charge and the inductive effect is called positive inductive effect (+I effect)
- 15. C : H C H H C H H H C X C : X C X C Y C : Y C Y C X C Y Standard -I Effect +I Effect C C C X C C C X -I Effect group (electron attracting/withdrawing group): NO2 > F > COOH > Cl > Br > I > OH > C6H5 +I Effect group (electron resealing group): (CH3)3C- > (CH3)2CH- > CH3CH2 - > CH3- C--C---C---X g---b---a -I Effect +I Effect
- 16. Electromeric effects: The effect which causes a temporary polarization in the substrate at the seat of a multiple bond by shift of an electron pair from one atom to other under the influence of an eletrophillic agent is called electrometric effect. When a double or triple bond is exposed to attack by an electrophillic agent, a pair of bonding electron is transferred completely from anoe atom to another. The atom that takes the electron pair becomes negatively charged and the other develops positive charge. or bond Temporary effect A B Electrophilic reagent added removed A B + - C C C C reagent E+ + electron
- 17. Mesomeric effect: When an electron withdrawing or pumping group is conjugated with a bond or a set of alternately arranged s and bonds, the electron displacement is transmitted trough the electrons in the chain. The electrons will be displaced to more electronegative atoms gives resonance in a molecule and thus develops polarity in a molecule. CH CH CH CH O CH CH CH CH OH2C + _ -M effect H2C CH CH CH NH2 CH CH CH NH2H2C + +M effect H2C -
- 18. Hyperconjugative effect: Hyperconjugation effect takes place through the interaction of s electrons of C-H bond with electrons of the double bond. CH CH2C H H H CH CH2C H H H + - * Greater the number of C-H bonds at a position to the unsaturated system, greater would be the electron release towards the terminal C (* mark) creating high electron density. C H H H C H H3C H C CH3 H3C H C> > > CH3 CH3 H3C 10 20 30
- 19. Types of Reactions • Addition reaction • Substitution reaction • Elimination reaction • Rearrangement reaction
- 24. Electrophilic Addition reactions in Conjugated Dienes Conjugated dienes are compounds having two double bonds joined by one s bond. Conjugated dienes are also called 1,3-dienes. 1,3-Butadiene (CH2=CH-CH=CH2) is the simplest conjugated diene. •A conjugated diene is more stable than an isolated diene because a conjugated diene has overlapping p orbitals on four adjacent atoms. Thus, its electrons are delocalized over four atoms.
- 25. Electrophilic Addition: 1,2- Versus 1,4-Addition The bonds in conjugated dienes undergo addition reactions that differ in two ways from the addition reactions of isolated double bonds. Electrophilic addition in conjugated dienes gives a mixture of products. Conjugated dienes undergo a unique addition reaction not seen in alkenes or isolated dienes. Electrophilic addition of one equivalent of HBr to an isolated diene yields one product and Markovnikov’s rule is followed. Electrophilic addition in conjugated dienes gives a mixture of products 1,2-addition product - addition at the 1- and 2-positions - direct addition 1,4-addition product - addition at the 1- and 4-positions - conjugate addition
- 26. Electrophilic addition of one equivalent of HBr in a conjugated diene affords two products. • The 1,2-addition product results from Markovnikov addition of HBr across two adjacent carbon atoms (C1 and C2) of the diene. • The 1,4-addition product results from addition of HBr to the two end carbons (C1 and C4) of the diene. 1,4-Addition is also called conjugate addition. • Addition of HX to a conjugated diene forms 1,2- and 1,4-products because of the resonance- stabilized allylic carbocation intermediate. • The ends of the 1,3-diene are called C1 and C4 arbitrarily, without regard to IUPAC numbering.
- 28. Kinetic Versus Thermodynamic Products The amount of 1,2- and 1,4-addition products formed in electrophilic addition reactions of conjugated dienes depends greatly on the reaction conditions When a mixture containing predominantly the 1,2-product is heated, the 1,4-addition product becomes the major product at equilibrium
- 29. Nucleophilic substitution is a fundamental class of substitution reaction in which an "electron rich" nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom attached to a group or atom called the leaving group. In nucleophilic substitution reactions, the C–X bond of the substrate undergoes heterolysis, and the lone-pair electrons of the nucleophile is used to form a new bond to the carbon atom A nucleophile is an the electron rich species that will react with an electron poor species. A leaving group , LG, is an atom (or a group of atoms) that is displaced as stable species taking with it the bonding electrons.
- 30. SN2 reaction: When the rate of a nucleophilic substitution reaction depends on both of the concentration of substrate and nucleophile is known as SN2 reaction. In SN2 reaction, a lone pair of electron from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step. Since two reacting species are involved, rate-determining step of the reaction, this leads to the name bimolecular nucleophilic substitution, or SN2. Kinetics: The rate of the reaction depends on the concentration of substrate and the concentration of nucleophile. Rate of reaction ∝ [Substrate] [Nucleophile]. Thus, the reaction is bimolecular i.e. two species are involved in the rate-determining step. Reaction order: The reaction is second order overall The SN2 reaction: Substitution, Nucleophilic, bimolecular
- 31. MECHANISM OF SN2 REACTION
- 32. MECHANISM OF SN2 REACTION • The nucleophile attacks the carbon bearing the leaving group from the backside. The nucleophile attacks the carbon at 180° to the leaving group and the leaving group is then pushed off the opposite side and the product is formed. • The bond between the nucleophile and the carbon atom is forming, and the bond between the carbon atom and the leaving group is breaking. The formation of the bond between the nucleophile and the carbon atom provides most of the energy necessary to break the bond between the carbon atom and the leaving group. • The transition state involves both the nucleophile and the substrate, in which the nucleophile and the leaving group are both bonded to the carbon atom undergoing attack so the carbon under nucleophilic attack is pentacoordinate. • Bond formation and bond breaking occur simultaneously in a single transition state, the SN2 reaction is a concerted reaction. • The configuration of the carbon atom becomes inverted during SN2 reaction.
- 33. STEREOCHEMISTRY OF SN2 REACTIONS In an SN2 reaction, the nucleophile attacks from the back side, that is, from the side directly opposite the leaving group and this attack causes a change in the configuration of the carbon atom so the configuration of the carbon atom becomes inverted which is known as Walden inversion because the first observation of such an inversion was made by the Latvian chemist Paul Walden in 1896.
- 34. Inversion of configuration can be observed when hydroxide ion reacts with cis-1-chloro-3-methylcyclopentane in an SN2 reaction Inversion of configuration can be also observed when the SN2 reaction takes place at a stereocenter (with complete inversion of stereochemistry at the chiral carbon center)
- 35. SN1 reaction: When the rate of a nucleophilic substitution reaction depends only the concentration of substrate molecule is known as SN1 reaction. In an SN1 reaction, there is loss of the leaving group generates an intermediate carbocation which is then undergoes a rapid reaction with the nucleophile. H3C C Br H3C H3C OH- OH- H3C C H3C H3C Kinetics: The rate of the reaction depends on the concentration of substrate but not the concentration of nucleophile. Rate of reaction ∝ [Substrate]. Thus, the reaction is uniimolecular i.e. one species is involved in the rate-determining step. Reaction order: The reaction is first order overall The SN1 reaction: Substitution, Nucleophilic, unimolecular
- 36. MECHANISM OF SN1 REACTION Step 1: Rate determining (slow) loss of the leaving group, LG, to generate a carbocation intermediate H3C C CH3 CH3 + OH- CH3 CHO CH3 CH3 H3C C OH H3C H3C (a) (b)(a) (b) Inversion (Predominates) Retension Step 2: Rapid attack of a nucleophile on the electrophilic carbocation to form a new σ bond H3C C Br H3C H3C H3C C CH3 CH3 + Br - + Carbonium ion
- 37. STEREOCHEMISTRY OF SN1 REACTIONS The carbocation has a trigonal planar structure and it may react with a nucleophile from either the front side or the back side: H3C C Br H3C H3C H3C C CH3 CH3 + Br - + OH OH With the tert-butyl cation it makes no difference but with some cations (optically active compounds), different products arise from the two reaction possibilities.
- 38. SN1 reaction proceeds with racemization In SN1 reaction, racemization (a reaction that transforms an optically active compound into a racemic form) takes place whenever the reaction causes chiral molecules to be converted to an achiral intermediate. The SN1 reaction proceeds with racemization because the intermediate carbocation is achiral and attacked by the nucleophile can occur from either side. The carbocation has a trigonal planar structure and is achiral. (S)-3-bromo-3-methylhexane (optically active) (S)-3-methyl-3-hexanol (R)-3-methyl-3-hexanol (optically inactive, a racemic form)
- 39. OH- This side open to attack This side shielded from attack (retention)(inversion)
- 40. OH- This side open to attack This side shielded from attack (retention)(inversion)
- 41. FACTORS AFFECTING THE RATES OF SN1 AND SN2 REACTIONS • The structure of the substrate • The concentration and reactivity of the nucleophile • The effect of the solvent • The nature of the leaving group. The effect of the structure of the substrate on SN2 reactions: In an SN2 reaction, the nucleophile attacks from the back side, that is, from the side directly opposite the leaving group. Substituents on or near the reacting carbon have a dramatic inhibiting effect. Simple alkyl halides show the following general order of reactivity in SN2 reactions: methyl > 1° > 2° >> 3° (unreactive) because the central carbon atom surrounded by bulky groups will be sterically hindered for SN2 reactions. A steric effect is an effect on relative rates caused by the space-filling properties of those parts of a molecule attached at or near the reacting site. Steric hindrance: the spatial arrangement of the atoms or groups at or near the reacting site of a molecule hinders or retards a reaction. Although most molecules are reasonably flexible, very large and bulky groups
- 42. H C X H H OH- H3C C X H3C H3C OH- Easy attack Difficult attack Methyl 1° 2° 3° Neopentyl
- 43. SN1 Reactions: In an SN1 reaction, there is loss of the leaving group generates an intermediate carbocation. The primary factor that determines the reactivity of organic substrates in an SN1 reaction is the relative stability of the carbocation that is formed. Organic compounds that are capable of forming relatively stable carbocation can undergo SN1 reaction at a reasonable rate. The stability order of carbocations is exactly the order of SN1 reactivity for alkyl halides. The relative stabilities of carbocations is 3° > 2° > 1° > methyl C R R R + C H H R +C R H R +> > 30 20 10 C H H H + Thus the order of reactivity in SN1 reactions: 3° > 2° > 1° > methyl (unreactive)
- 44. Thus, the order of reactivity in SN2 reactions: methyl > 1° > 2° >> 3° (unreactive) and in SN1 reactions: 3° > 2° > 1° > methyl (unreactive)
- 45. Effect of the nature and the concentration of the nucleophile SN1 reactions: Neither the concentration nor the structure of the nucleophile affects the rates of SN1 reactions since the nucleophile does not participate in the rate- determining step. SN2 reactions: The rates of SN2 reactions depend on both the concentration and the structure of the nucleophile. A stronger nucleophile attacks the substrate faster. A strong nucleophile as well as high concentration of the nucleophile always favor the SN2 reactions A negatively charged nucleophile is always a more reactive nucleophile than its conjugate acid. HO– is a better nucleophile than H2O; RO– is a better nucleophile than ROH. “Nucleophilicity” measures the affinity (or how rapidly) of a Lewis base for a carbon atom in the SN2 reaction (relative rates of the reaction). “Basicity”, as expressed by pKa, measures the affinity of a base for a proton (or the position of an acid-base equilibrium).
- 46. SOLVENT EFFECTS ON SN2 REACTIONS Protic Solvents: The solvent molecule has a hydrogen atom attached to an atom of a strongly electronegative element e.g. hydroxylic solvents such as alcohols and water Molecules of protic solvents form hydrogen bonds with the nucleophiles and slows down the rate of SN2 reactions. Polar Aprotic Solvent: Aprotic solvents are those solvents whose molecules do not have a hydrogen atom attached to an atom of a strongly electronegative element. Polar aprotic solvents are especially useful in SN2 reactions because polar aprotic solvents do not solvate anions to any appreciable extent because they cannot form hydrogen bonds. The rates of SN2 reactions generally are vastly increased when they are carried out in polar aprotic solvents.
- 47. (DMF) (DMSO) (DMA) Polar aprotic solvents dissolve ionic compounds, and they solvate cations very well. A sodium ion solvated by molecules of the protic solvent water
- 48. SOLVENT EFFECTS ON SN1 REACTIONS Since SN1 reaction involves the the formation of carbocation intermediate in the rate determining step, anything that can facilitate will speed up the reaction. Polar protic solvent will greatly increase the rate of ionization of an alkyl halide in any SN1 reaction. Polar protic solvents solvate cations and anions effecttively. Cations are solvated chiefly through unshared pares of elctrons: anions are solvated chiefly through hydrogen bonding. Solvation stabilizes the transition state leading to the intermediate carbocation and halide ion more it does the reactants. Thus SN1 reactions proceed more rapidly in water, alcohols, and mixture of water and alcohols than in aprotic solvents like DMF and DMSO
- 49. Elimination reactions: Two or four atoms or groups attached to the adjacent carbon atoms in the substrate molecule are eliminated to form a multiple bond. Elimination reactions are important as a method for the preparation of alkenes. The two most elimination reactions are: • Dehydration (-H2O) of alcohols, and • Dehydrohalogenation (-HX) of alkyl halides. Dehydrohalogenation involves the elimination of the halogen atom and of a hydrogen atom from a carbon adjacent to the one losing the halogen. The reagent required is a base, whose function is to abstract the hydrogen as a proton. Dehydrohalogenation is a b elimination or1,2-elimination reaction because the carbon holding the halogen is designated as a or 1-carbon and elimination involves the loss of b or 2-hydrogen from b or 2-carbon adjacent carbon holding the halogen. There are three fundamental events in these elimination reactions: • Removal of a proton • Formation of the CC bond • Breaking of the bond to the leaving group R CH CH2 X HOH- R CH CH2 H2O + X- + 12
- 50. How does such an elimination reaction generate a double bond? Regardless of the exact mechanisms, • Halogen leaves the molecule as halide ion and hence must take its electron pair along. • Hydrogen is abstracted by the base as a proton and hence leave its electron pair behind. • It is this electron pair that is available to form the second bond, the bond between the carbon atoms. R CH CH2 X HOH- R CH CH2 H2O + X-+ Types of elimination reactions: • Bimolecular Elimination Reaction (E2) • Unimolecular Elimination Reaction (E1)
- 51. Bimolecular Elimination Reaction (E2): E2 reactions proceed by second order kinetics and the reaction involves in a single step: Base pulls a proton away from the carbon; simultaneously a halide ion departs and the double bond forms between the carbons. Halogen takes its electron pair along with it and hydrogen leaves its electron pair behind to form the double bond. R CH CH2 X R CH CH2 H R C H CH2 X H OH Transition state OH- The base begins to remove a proton from the β-carbon using its electron pair to form a bond to it. At the same time, the electron pair of the β C−H bond begins to move in to become the π bond of a double bond, and the bromide begins to depart with the electrons that bonded it to the α carbon. Partial bonds now exist between the oxygen and the β hydrogen and between the α carbon and the bromine. The carbon-carbon bond is developing double bond character. Now the double bond of the alkene is fully formed
- 52. Orientation of elimination reactions CH3 CH2 CH2 Br KOH (alc) CH3 CH CH2 Dehydrohalogenation yields a single alkene C H b Dehydrohalogenation yields a mixture of alkenes corresponding to the loss of any one of the b-hydrogen CH3 CH2 CH CH3 KOH (alc) CH3 CH CH Br CH3 CH3 CH2 CH CH2 + CH3 CH2 C CH3 KOH (alc) CH3 CH C Br CH3 CH3 CH2 C CH2 + CH3 CH3 CH3 In a mixture of alkenes which one is the preferred product and why?
- 53. Zaitsev's rule, Saytzeff's rule or Saytsev's rule named after Alexander Mikhailovich Zaitsev is a rule that states that if more than one alkene can be formed by an elimination reaction, the more stable alkene is the major product. When an alkylhalide has two or three b carbons, a mixture of isomers is possible and the more substituted alkene is the major product because the compound that has a more highly substituted C=C double bond is more stable due to the electron donating properties of the alkyl group. • The major product will be the most highly substituted alkene, the product with the fewest H substituents on the double bonded carbons
- 54. Unimolecular Elimination Reaction (E1): E1 reaction proceeds by first order kinetics and involves of two steps: Step 1: Substrate undergoes heterolysis to form halide ion and carbocation Step 2: The carbocation rapidly loses the proton to the base and forms alkenes CH3 C X CH3 CH3 C X- CH2 H3C CH3 + H Slow C X- CH2 H3C CH3 + H OH- Slow C CH2 H3C CH3 H2O + X-+
- 55. E1 reactions accompained by rearrangement, where structure permits CH3 C C CH3 CH3 CH3 H CH3 C CH CH3 CH3 CH3 Br CH3 C C CH3 CH3 CH3 H CH3 C C CH3 CH3 H CH3 CH3 C C CH2 CH3 H CH3 CH3 C C CH3 CH3 CH3 2° carbocation Methyl shift 3° carbocation Major productMinor product
- 56. SN2 VERSUS E2 Since eliminations occur best by an E2 path when carried out with a high concentration of a strong base (and thus a high concentration of a strong nucleophile), substitution reactions by an SN2 path often compete with the elimination reaction. (a) When the nucleophile (base) attacks a β carbon atom, elimination occurs. (b) When the nucleophile (base) attacks the carbon atom bearing the leaving group, substitution results. Primary halides: substitution is favored, Secondary halides: elimination is favored and Tertiary halides: no SN2 reaction, elimination reaction is highly favored
- 57. THE NATURE OF THE LEAVING GROUP The best leaving groups are those that become the most stable ions after they depart. Most leaving groups leave as a negative ion and the best leaving groups are those ions that stabilize a negative charge most effectively. The leaving group begins to acquire a negative charge as the transition state is reached in either an SN1 or SN2 reaction SN1 Reaction (rate-limiting step) SN2 Reaction Stabilization of the developing negative charge at the leaving group stabilizes the transition state (lowers its free energy) lowers the free energy of activation and increases the rate of the reaction. The effect of the leaving group is the same in both SN1 and SN2: R–I > R–Br > R–Cl
- 58. Rearrangement Reaction A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. The reactions which proceed by a rearrangement or reshufling or shifting of atoms/groups from one position to another in the molecule to produce a structural isomer of the original substance are called rearrangement reaction. CH3 CH2 CH2 CH3 CH3 CH CH3 CH3 n-Butane iso-Butane • Intramolecular rearrangement • Intermolecular rarrangement
- 59. Hofmann rearrangement • Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. • Primary amide is transferred into an intermediate isocyanat in the presence of bromine with sodium hydroxide • The intermediate isocyanate is hydrolyzed to a primary amine giving off carbon dioxide.
- 61. Beckmann Rearrangement • When ketoxime is treated with an acidic catalyst such as H2SO4, H3PO4, SOCl2, PCl5 etc., it is converted into a substituted amide. • The reaction mechanism of the Beckmann rearrangement is generally believed to consist of an alkyl migration with expulsion of the hydroxyl group to form a nitrilium ion followed by hydrolysis CH3 C CH2 CH3 N OH C CH2 CH3 HN CH3 O PCl5 R C R' N OH R C R' N Cl C R' NR + H2O: C R' NR OH H +C R' NR OHC R' NHR O PCl5
- 62. Fries rearrangement is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of lewis acids OH OCOR OH OH RCOCl AlCl3 COR COR + O C R O AlCl3 O C R O O C R O AlCl3 AlCl3 O C R O AlCl3 O C R O AlCl3 O C R O AlCl3 H O C R O AlCl3 O C R O H2O H + _ _ + _ + _ ++ _ + - H+ _ Mechanism
- 63. Mechanism of Fries rearrangement • Lewis acid, AlCl3 co-ordinates to the carbonyl oxygen atom of the acyl group because this oxygen atom is more electron rich than the phenolic oxygen atom • Polarization of the bond between the acyl residue and the phenolic oxygen atom and AlCl3 group rearranges to the phenolic oxygen atom. • Generation of a free acylium carbocation which reacts in a classical electrophilic aromatic substitution with the aromat. • The abstracted proton is released as hydrochloric acid where the chlorine is derived from aluminium chloride. • The orientation of the substitution reaction is temperature dependent. A low reaction temperature favors para substitution and with high temperatures the ortho product
