Patente de insectoslunes 07082018
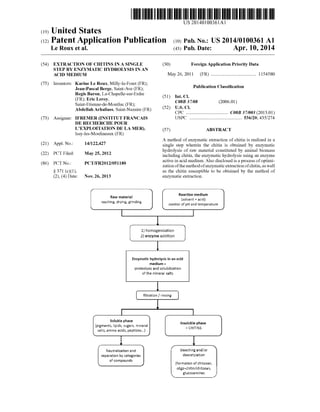
- 1. (19) United States (12) Patent Application Publication (10) Pub. No.: US2014/0100361 A1 US 201401.00361A1 Le Roux et al. 43) Pub. Date: Apr. 10, 20149 (54) EXTRACTION OF CHITINS IN A SINGLE (30) Foreign Application Priority Data STEP BY ENZYMATIC HYDROLYSIS IN AN ACID MEDIUM May 26, 2011 (FR) ....................................... 115458O (75) Inventors: Karine Le Roux, Milly-la-Foret (FR): O O Jean-Pascal Berge, Saint-Ave (FR): Publication Classification RiseBy hapeleurline (51) Int.Cl./ . s COSB 37/08 (2006.01) Saint-Etienne-de-Montluc (FR); (52) U.S. Cl Abdellah Arhaliass, Saint-Nazaire (FR) CPC .................................... C08B 37/003 (2013.01) (73) Assignee: IFREMER (INSTITUT FRANCAIS USPC ............................................. 536/20: 435/274 DE RECHERCHE POUR LEXPLOITATION DE LA MER), (57) ABSTRACT Issy-les-Moulineaux (FR) A method ofenzymatic extraction ofchitin is realized in a (21) Appl. No.: 14/122,427 single step wherein the chitin is obtained by enzymatic 1-1. hydrolysis of raw material constituted by animal biomass (22) PCT Filed: May 25, 2012 including chitin, the enzymatic hydrolysis using an enzyme act1Ve 1n ac1d medium. Also d1SCIOSed 1S arOceSS Of Ot1m1(86). PCT No.: PCT/FR2O12/05118O ive in acid medium. Also disclosed is a p foptimi S371 (c)(1), (2), (4) Date: Nov. 26, 2013 Raw rateria Washing, dying, giding zation ofthe methodofenzymaticextractionofchitin,as well as the chitin susceptible to be obtained by the method of enzymatic extraction. Reaction edium sowent + acid control ofpH and temperature homogenization 2 enzyme additio: Soisbie phase pigments, tigids, Sigars, i.e.ai Saits, airino acids, peptides...} Neutralizatio aid separatios by categories of compouds Enzymatic hydroEysis inan acid nedin = proteoysis and Scitizatio: of the litera sats filtration f rising insibie phase = CH3S bleaching aidfor deacetylation foration of chitosan, oligo-chiti chitosai, gucosamines
- 2. Patent Application Publication Apr. 10, 2014 US 2014/0100361 A1 Reaction medium {so went + acid} contro ofpH and temperatuie Raw materia Washing, dying, giding homogenization 2} enzyme addition Enzymatic hydrolysis in an acid medium = proteoiysis and soiuxilization of the irrera sats fitration f rising Soluble phase pigments, tipids, Sugars, minera: saits, aimino acids, peptides...} insolubie phase = CHENS bleachingandfor deacetylation Neutralization aid Separation by categories of compounds r iformation of chitosan, oligo-critifchitosa, gucosamines F.G. 1
- 3. US 2014/0100361 A1 EXTRACTION OF CHTINS IN A SINGLE STEP BY ENZYMATIC HYDROLYSIS IN AN ACID MEDUM FIELD OF INVENTION 0001. The present invention relates to the field of the recovery ofbiomass, preferably animal biomass, more pref erably marine and/or entomological biomass. In particular, the present invention relates to a method for the enzymatic extraction of chitin in a single step, from animal biomass elements comprising chitin, preferably from marine and/or entomologicalby-products,usinganactiveenzymeinanacid medium. BACKGROUND OF INVENTION 0002 The production and consumption of marine prod ucts, and particularly of crustaceans, notably prawns, is increasing each year. By-products (heads and shells) gener ally represent more than 50% ofthe fresh weight ofcrusta ceans. Theusethereofisthus amajorissuegiventheVolumes involved and also the slow natural biodegradability thereof. Chitin is the main product derived from these by-products. 0003 Moreover, entomophagy is a common dietary prac ticeinsomecountries whichistendingtodevelop worldwide. Indeed, insects represent an advantageous dietary resource due to the nutritional qualities thereof. Furthermore, insect production offers a very advantageous environmentally friendly alternative compared to the production ofotherani mal proteins. The by-products obtained from insect protein production include chitin-rich shells, as for crustaceans. 0004 Chitin is the second most plentiful polysaccharide on the surface ofthe Earth after cellulose. It does not have a single chemical structure, but several, since it includes polysaccharides consisting of N-acetyl-B-D-glucosamine units and D-glucosamine units. 0005 Chitinpartiallyformstheexoskeleton ofinsectsand crustaceans and the wall of fungi and bacteria. Chitin thus represents 20 to 30% of the shells of crustaceans. Besides chitin, the exoskeleton ofcrustaceans contains 20 to 40% of proteins, 30 to 60% ofmineralsand 0to 14% offat (Waldeck J. Daum G., Bisping B. and Meinhardt F., Appl. Env, Micro biol., 2006, 72 (12), 7879-7885). Chitin thus represents 3 to 60% oftheshellsofinsects. Besideschitin, theexoskeletonof insects contains 20 to 80% ofproteins, 1 to 20% ofminerals and 10 to 50% offat (“Forest insects as food: humans bite back. Proceedings ofa workshop onAsia-Pacific resources andtheirpotential fordevelopment, 19-21 Feb. 2008,Chiang Mai, Thailand FAO). Whether for crustaceans or for insects, the proportions of the various constituents vary according to the species, age, genus and may fluctuate according to the seasons and environmental conditions. Chitin extraction conditions should thus be adapted accord ing to the raw material used (Tolaimate A. Desbrieres J. Rhazi M.andAlaguiA., Polymer, 2003, 44(26), 7939-7952). 0006 Chitin is found incrustaceanandinsectby-products in the form ofchitin/proteinS/minerals complexes. It is usu ally extracted in two “chemical extraction' steps: 0007 demineralization by means ofacid hydrolysis, to remove minerals; and 0008 deproteinization by means ofbase hydrolysis, to remove proteins. 0009 Chitin extraction from marine by-products is cur rently carried out on an industrial scaleby means of"chemi Apr. 10, 2014 cal extraction'. Chitin extraction from insects has not been very developed to date but has already been the subject of studies, essentially using a chemical process (cicada chitin: Sajomsang W. and Gonil P. Mat. Science Engineering C, 2010, 30(3), 357-363; silkworm pupa chitin: Paulino A., Simionato J. Garcia J. and Nozaki J. Carbohydrate Poly mers, 2006, 64, 98-103; bumblebee chitin: Majtan J., Bil ikova K. Markovic O. Grog, J., Kogan G. andSimuth J., Int. J. Biol. Macromol., 2007, 40,237-241). 0010. A third optional bleaching step, for example using Sodium hypochlorite, is frequently used to remove residual pigments. Washing operations, generally with water, are required between these various steps. 0011 Chitincan thenbereadilydeacetylated, forexample using sodium hydroxide, to produce chitosan. 0012 Chitin is conventionally extracted for a wide range ofapplications: medical,pharmaceutical, dietary, food,tech nical (water filtration and depollution), etc. Indeed, chitin, chitosan and the derivatives thereof, particularly the oligo mers thereof, are biocompatible, biodegradable and non toxic. Thetype ofapplication depends on the physicochemi cal characteristics of chitin and the derivatives thereof. In particular, chitosan may be particularly used for producing mulching film, Stomach protection gels, but also for active ingredient encapsulation, waste water filtration, cartilage replacement, tissue regeneration, etc. 0013 Industrial chitin extraction from marine by-prod ucts is essentially implemented in emerging countries. Con ventional chemical extraction uses large quantities of reagents (essentially hydrochloric acid, Sodium hydroxide andbleachingagents)whichareharmful foroperators,equip mentandthe environment. Furthermore, thebasicdeprotein ization step is generally performed hot and thus requires a high energy input. Moreover, the washing steps give rise to very large volumes ofpolluted effluents, which are techni cally difficult and expensive to recycle. 0014. Oneoftheproblems associated with currentextrac tion methods is thepossibility ofchitin being denatured dur ing the process (Crini G., Badot P. and Guibal E., Chitine et Chitosan. Du polymered l'application, 2009, Presses Uni versitaires de Franche-Comté). 0015 Studies have shown that chitin could be extracted using biological methods, notably by means of enzymatic extraction or microbiological fermentation, particularly for the deproteinization step. 0016. Of the research on the fermentation process, the studies conducted by Beaney use the exoskeleton of the Nephrops norvegicus prawn as the study Substance (Beaney P. Lizardi-Mendoza.J. andHealy M.,J. Chem. Tech. Biotech., 2005,80, 145-150). In this study, chitinisextractedby means oflactic fermentation in thepresenceofbacterialstrains for5 days at 30°C. Theacidification ofthe medium, due to lactic acid production by the bacteria, results in partial demineral ization while the bacteria carry out deproteinization. In this study, the pH decreases to a value of 3.5 after 7 days of fermentation. However, under these conditions, the chitin extracted still contains 13% ofproteins and 14% minerals. A purer chitin may then be obtained by performing further chemical treatments. This typeofmethodis thus not suitable fordirectlyobtainingahigh-quality chitin,limitingtheappli cations thereof. 0017. A further microbial fermentation study was con ducted to extractchitin from redcrab shellsby co-fermenting the shells in the presence oftwo bacteria: firstly, Lactobacil
- 4. US 2014/0100361 A1 lus paracasei tolerans KCTC-3074, which is a lactic acid producing bacterium, and secondly Serratia marcescens FS-3,which isanextracellularprotease-producingbacterium (JungW.,JoG., Kuk.J., Kim K. andPark R. Appl. Microbiol. Biotechnol., 2006, 71,234-237). Co-fermentation was main tained for7 days at30° C. and resulted in a demineralization rate of97.2% and a deproteinization rate of merely 52.6%. Thechitin obtainedwas notcharacterisedin this studybutthe low deproteinization rate is limiting in terms of the use thereof. 0018. A further microbial fermentation study was con ducted by the same team, with a bacterial strain producing proteases for deproteinizing and demineralizing marine by products (Jo G., JungW. KukJ. Oh K. KimY. and Park R. Carbohydratepolymers, 2008, 74, 504-508). A fermentation testwasconductedfor7daysat30°C.,in thepresenceof10% of bacterial strain, and resulted in a deproteinization and demineralization rate of 84% and 47%, respectively. As above, the demineralization is due to the pH decrease over time (pH 5.6 after 7 days of fermentation), associated with bacterial acid production. The low degree of purity of the chitins obtained is limiting in terms of the applications thereofand this method, like the previous method, involves the drawback ofrequiring a very long reaction time. 0019. The best demineralization and deproteinization yields by means of a two-step fermentation process were obtained by Waldeck (Waldeck J. Daum G., Bisping B. and Meinhardt F., Appl. Env, Microbiol., 2006, 72 (12), 7879 7885).Afterfermentingfor6daysfrom42to55°C.,followed by a 3hour lactic acid treatment, the residual protein content is less than 10% and the demineralization rate is equal to 98.8%. As in the study by Jo et al., the reaction time is relatively long. 0020 Chitin extraction by means ofa fermentation pro cess thus results in a chitin with a higher residual protein content than in the case of chemical extraction and further treatments are frequently required to improvethe demineral ization. Furthermore,thereactiontimesare muchlongerthan with the chemical process. 0021 Chitin extraction by means ofa biological process may also be performed using enzymatic extraction. 0022. A method for extracting chitin comprising the removalofproteinsbymeansoftheenzymaticactivity offish viscera was proposed in the international patent application WO 86/06082. In particular, the method described in this patent application comprises the extraction of chitin from prawn shells by means ofdemineralization with an acid fol lowed by deproteinization using fish viscera, optionally pre ensiledat pH 1.2-2.5. The raw material, i.e. the prawn shells, is first ensiled in a Sulphuric acid solution. Ensiling makes it possible to store the raw material before use and enables the demineralization thereof. Secondly,thepre-ensiled shellsare placed in contact with fish viscera for deproteinization. The characteristics of the chitin obtained using this two-step method are not specified. 0023. Enzymatic extraction may also be performed using apurifiedenzyme,generallyaproteolyticenzyme.This is,for example, the case in the study conducted by N. Gagné, with the use ofchymotrypsin orpapain for extracting chitin from prawn shells (Gagné N. “Production ofchitin and chitosan from crustacean wasteandtheiruseasfoodprocessing aid', 1993 McGill University—Montreal, doctoral thesis). Aftera conventional chemical demineralization step, the proteins present are hydrolysed by the enzymes. The optimal depro Apr. 10, 2014 teinization conditionsparticularly involveapH of8.0-8.7 for chymotrypsin and papain. Under the conditions used, the residual protein content is very low (1.3% and 2.8% forchy motrypsin and papain, respectively). 0024. The same type ofmethod was used by Synowiecki and Al Khateeb for conducting the enzymatic digestion of prawn shells, previously demineralized with hydrochloric acid, usingalcalase, at 55° C. and pH8.5 (Synowiecki J. and Al Khateeb, Food Chemistry, 2000, 68, 147-152). 0025. A method for producing chitin involving an enzy matic hydrolysis step has been patented (CN1715255). This method offers a general approach for processing the raw material sincecompounds otherthan chitinarealso extracted from prawn shells. In particular, this method comprises an enzymatic hydrolysis step followed by solvent extract. The solidportionobtained is thenplacedinthepresenceofhydro chloricacidtoperform demineralizationandfinishextracting chitin. 0026. All the enzymatic chitin extraction methods cur rently described involvean independentconventional chemi cal demineralization step, before or after the enzymatic hydrolysis step. In this way, even ifthe deproteinization step is carriedoutusingabiological method,there is still achemi calstep requiring washing operations andproducingpolluted effluents and liable to affect the properties of the extracted chitin. 0027. The current methods are thus not satisfactory and thereisaneedforchitinextractionmethodswhicharesimple, rapid, efficient, inexpensive and more environmentally friendly. These methods should be suitable for producing chitins wherein thepurity iscompatible with usein the food, dietetic or cosmetic industries. Furthermore, the chitins pro ducedshould meetthespecifications requiredtobeprocessed into chitosan, oligo-chitosan orglucosamines,etc. Inparticu lar, the degree of polymerization of the chitin should be sufficiently high and it should not be denatured during the process. 0028 Moreover, further compounds can potentially be recovered using crustacean and insect by-products, particu larly in nutraceuticals, dietetics or cosmetics. Indeed, these marine and insect by-products contain soluble compounds Suchas lipids,pigments, Sugars, mineral salts, aminoacids or peptides. Targeted extractions of these soluble compounds have been developed, such as for example the extraction of pigmentsand inparticularofastaxanthin which is usedin the food industry (U.S. Pat. No. 7,241,463). However, these methods targeted on Soluble compound extraction require further steps to conduct chitin extraction. 0029. In current chitin extraction methods, chitin is obtained in Solid form and the liquid extraction phases, con taining soluble compounds of potential interest, are not recovered.Thislackofrecovery canparticularlybeexplained by the poor quality ofthe Soluble compounds present in the liquid phases. Indeed, in these chitin extraction methods, soluble compounds are frequently degraded due to the rela tively severe conditions used. 0030 Therefore, there is a need for chitin purification usinga methodthatrespects theinitial structurethereofmore and is also suitable forbeingassociated with soluble product co-extraction. 0031. TheApplicantconducted research inordertoobtain full recovery of the portions of animal biomass containing chitin, notably full recovery of marine by-products, in par ticular crustacean shells and entomological biomass, in par
- 5. US 2014/0100361 A1 ticularinsectshells. Inparticular,co-extraction methods were studied with a view to the advantages thereof in relation to targeted extractions. SUMMARY 0032. The invention thus relates toa method fortheenzy maticextraction ofchitin,characterisedin thatsaid methodis carried out in a single step, hereinafter referred to as the “single step', wherein chitin is obtained by the enzymatic hydrolysis ofanimal biomass comprising chitin, said enzy matic hydrolysis using an active enzyme in an acid medium. 0033 Accordingto oneembodiment, saidsinglestepisan enzymatic hydrolysis intended for deproteinizing and dem ineralizing marine by-products simultaneously. 0034. According to one embodiment, saidactive enzyme in an acid medium is a protease having a broad spectrum of activity inan acid medium, preferably pepsin ora stableacid protease. 0035. Accordingto oneembodiment,theenzyme concen trationused forhydrolysis is 0.1 to 75%,preferably5 to30%, more preferably from approximately 23 to approximately 27% in weight relative to the weight of the protein mass estimated in the raw material. 0036. According to one embodiment, the acid medium is obtained by means ofthe presence of an acid, preferably a dietary acid, morepreferably phosphoric acid orformic acid. 0037 Accordingtooneembodiment, saidanimalbiomass comprising chitin comprises marine by-products, preferably marine by-products obtained from crustaceans, preferably prawns, crabs orkrill, orfrom cephalopods,preferably squid or cuttlefish. 0038 Accordingtooneembodiment, saidanimalbiomass comprising chitin comprises insect by-products, preferably insect by-products obtained from beetles orhymenoptera. 0039. According to one embodiment, said methodfurther comprises operationsforwashing,dryingand/orgrindingthe raw material, preferably water washing, cold drying and/or grinding operations resulting in fragments less than 1 mm in S17C. 0040. According to one embodiment, said methodfurther comprisesreactionmediumtreatmentoperationsattheendof the enzymatic hydrolysis, said operations comprising opera tionsforseparatingtheSolidandliquidphases, rinsingand/or dryingtheinsolubleportion,preferably operations consisting offiltration, rinsing with waterand/oroven-drying. 0041. The invention also relates to a method for optimis ing said method forthe enzymatic extraction ofchitin, char acterized in that said optimization method comprises at least one ofthe following steps: 0042 a) selecting the pH oftheacid medium in the range pH-0-2, preferably pH-0-1.5, preferably pH-0-1,82 82 82 wherepH isthepHatwhichtheenzymeexhibits maximum activity, 0043 b) selecting the temperature ofthe acid medium in therangeTit.0-20°C.,preferablyTit.0-15°C., preferably T+0-10° C., where T is the temperature at which the enzyme exhibits maximum activity, 0044 c) determining the mineral and protein content of the raw material, 0045 d) calculating the acid concentration to be used in the reaction medium, accordingto the mineral content ofthe raw material, itbeing understood that, according to one pre Apr. 10, 2014 ferred embodiment, the pH is selected such that the reaction medium is maintained throughout the enzymatic hydrolysis atthe pH selected in step a). 0046 e) calculating the proportion ofenzyme to be used with respect to the protein content ofthe raw material, 0047 f)determiningthe reaction timeforobtaining chitin orthe chitin derivatives sought. 0048. The invention further relates to chitin that can be obtained by means ofthe method accordingto the invention. 0049. The invention also relates to chitosan that can be obtained by deacetylating chitin according to the invention. 0050. Theinventionalso relatesto acompositioncompris ing chitin accordingto the invention and/or chitosan accord ing to the invention. 0051. Theinvention also relates to a pharmaceutical com position comprising chitin according to the invention and/or chitosan according to the invention, a cosmetic composition comprising chitin according to the invention and/or chitosan according to the invention, a medical device comprising chitinaccordingtotheinventionand/orchitosanaccordingto the invention. 0.052 Theinventionalso relatestoafoodproduct,a nutra ceutical composition, a dietetic composition, a food Supple ment or a functional food comprising chitin according to the invention and/or chitosan according to the invention. 0053. Theinventionalso relatesto acompositioncompris ing chitin accordingto the invention and/or chitosan accord ing to the invention for the use thereofin water treatment, filtration and/or water depollution. 0054 The invention also relates to a texturingagent com prising chitin according to the invention and/or chitosan according to the invention. DEFINITIONS 0055. In the present invention, the following terms are defined as follows: 0056 “Chitin’ refers to N-acetyl-glucosamineandglu cosamine polysaccharides. 0057 "Chitosan” refers to chitin deacetylation prod ucts. The borderline between chitosan and chitin con sists ofa 50% degree ofacetylation: below, the com pound is called chitosan, above, chitin. 0.058 Animal biomass” refers to all organic matter of animal origin. 0059) “Marineby-products” refers to parts not used by the food industry in marine products, particularly crus tacean shells and heads. 0060 “Entomological by-products”or“insectby-prod ucts” refers to parts not used by the food industry in entomological products, particularly insect shells and heads. 0061 “Degreeofpolymerization”referstothelengthof apolymerchain,particularly chitin.Thedegree ofpoly merization consists of the number of monomer units forming the polymer chain. 0062 “Crystallinity index' refers to the proportion of material found in the crystalline state. 0063 “Demineralization” referstoamethodforremov ing minerals. 0.064 “Deproteinization” refers to a method for remov ing proteins. 0065 “Depolymerization” referstothereductionofthe length ofthe polymeric chain ofchitin.
- 6. US 2014/0100361 A1 0066 “Deacetylation” refers to the removal of acetyl groups and corresponds to the transition from chitin to chitosan. 0067 “Moisturecontent” refers to the mass percentage ofwater contained in a sample. 0068 “Proteincontent” refers to the masspercentageof protein contained in a sample. 0069. “Mineral content” refers to the mass percentage ofminerals contained in a sample. 0070 "Chitin content” refers to the mass percentage of chitin contained in a sample. 0071 "Approximately,placedbeforea number, means more or less 10% ofthe nominal value ofthe number. 0072 Unless specified otherwise, the percentages are mass percentages. DETAILED DESCRIPTION 0073. The present invention relates to a method for the enzymatic extraction of chitin in a single step, from a raw material obtained from animal biomass and comprising chitin,preferably a raw material made ofmarine by-products and/orentomologicalby-products, usingan activeenzyme in an acid medium, preferably a protease, the acid used being preferably a dietary acid, this method also being suitable for extracting soluble compounds such as lipids, pigments, Sug ars, mineral salts, amino acids orpeptides. 0074. In the present invention, the two key steps of the conventional method forextracting chitin, i.e. demineraliza tion in an acid medium and deproteinization in an alkaline medium, are merged into a single step. This merging into a single step is enabled through the use ofan enzyme wherein the optimal activity pH is acidic: the enzyme performs the deproteinization, while the acidic pH makes it possible to carry outthe demineralization simultaneously. 0075. The method according to the invention only com prising a single key step, it offers the advantage ofreducing rinsing-related material losses between the two steps ofthe conventional method. This method also makes it possible to decrease the reagentand solvent consumption and limit pol luted effluent production. This method is thus both inexpen sive and environmentally friendly. 0076. Theconditions used in the method according to the present invention are such that the biological activity of chitins and the native structure thereofare preserved better than in the extraction methods existing to date. 0077. The method according to the present invention offers the advantage ofenabling destructuration ofthe crus tacean and/or insect by-product matrix by separating chitin, proteins and minerals, these three major constituents being initially strongly linked. 0078. The method according the invention comprises an enzymatic hydrolysis step in an acid medium performing demineralization and deproteinization simultaneously. The minerals and proteins are detached from the solid phase and carried in the liquid phase. 0079 According to one embodiment, the method accord ing to the present invention may comprise, in addition to the enzymatichydrolysisstepinanacid medium,preparationand processing operations: 0080 preparing the raw material, 0081 preparing a reaction medium according to the optimal conditions for enzymatic activity and compris ing at least one acid, Apr. 10, 2014 0082 mixing the raw material prepared in the reaction medium, homogenizing and adding enzyme, 0.083 enzymatic hydrolysis step with controlled tem perature, pH and stirring: I0084 simultaneous deproteinization and demineraliza tion reactions foran optimized time, 0085 separating the soluble and insoluble portions of the “reaction liquor. I0086) washing the “insoluble' portion, drying and packaging, 0.087 optionally, characterising the extracted products. 0088 Raw Material I0089. The term "raw material', according to the present invention,denotestheanimalbiomasscomprisingchitinused forextracting chitin, preferably marineby-products used for extracting chitin and/or entomological by-products used for extracting chitin. 0090 According to one embodiment, the raw material used in the method according to the present invention com prises marine by-products, preferably crustaceans, prawns, crabs, krill, more preferably crustacean shells and heads: accordingto oneparticularembodimentofthe invention, the raw material is obtained from cephalopods, preferably squid orcuttlefish. 0091. According to one embodiment, the raw material used in the method according to the present invention com prises entomological by-products, preferably from beetles such as the Tenebrio molitorbeetle, hymenoptera suchasthe Hermetia illucens fly, more preferably insect shells and heads. 0092. The raw material preparation operation should be suitable for retaining the qualities thereofwhile meeting the requirements ofthe method. 0093. According to oneembodimentofthe presentinven tion,theraw materialpreparation comprises cleaning, drying and/orgrinding operations. 0094. According to one embodiment, the raw material is cleaned with water. 0.095 According to one embodiment, the raw material is driedfrom 1 hourto36hourspreferably forapproximately 18 hours,preferablyinventilatedair,preferablyata temperature of 5 to 35° C., more preferably of approximately 12° C. According to one embodiment, the raw material is ground to obtain fragments having a maximum diameter equal to approximately 10mm,preferably havingadiameterlessthan approximately 1 mm. 0096. According to one embodiment, the raw material, preferably prepared by cleaning, drying and grinding, is stored prior to extraction at a temperature between -30 and -10°C., preferably at -20°C., preferably limiting the pres ence ofoxygen. 0097. Reaction Medium 0098. The term “reaction medium', according to the present invention, denotes the medium wherein the enzy matic hydrolysis reaction in an acid medium takes place. 0099. The reaction medium preparation should account forthe enzyme activity conditions used Such as thetempera ture, solventandpH. Theselectionoftheseconditions makes it possible to optimize the reaction time and yields. 0100. Accordingtooneembodiment,thereactionmedium is maintained during the enzymatic hydrolysis at a tempera turebetween2 and80°C., preferablybetween35 and45° C., morepreferably fromapproximately37 toapproximately 40° C.
- 7. US 2014/0100361 A1 0101. According to one embodiment, the temperature of the reaction medium is adapted to the enzyme used so that saidenzymehasaquasi-optimalactivitythroughouttheenzy matic hydrolysis. 0102) Accordingtooneembodiment,thereaction medium is maintained during the enzymatic hydrolysis at a tempera tureintherangeTit.0to20°C., preferablyTit.0to 15°C., preferably T0 to 10°C., where T is the temperature at which the enzyme exhibits maximum activity. The tempera tureselectedshould notinducethedegradation oftheenzyme orinhibittheactionthereof.Advantageously,thetemperature ofthe reaction medium is less than T. So as to limitenergy consumption. 0103) According to one embodiment, the pH ofthe reac tion medium is 0.5 to 6.5, preferably from 1.8 to 3.8, more preferably from approximately 1.9 to approximately 2.1. If the enzyme is pepsin, the pH of the reaction medium is preferably from approximately 1.9 to approximately 2.1. 0104. According to one embodiment, the pH ofthe reac tion medium is acidic and the value thereofis adapted to the enzyme used so that said enzyme has an optimal activity. 01.05 tion medium is in the range pH-t2, preferably pH-t1.5. preferably pH-1, where pH is the pH at which the enzyme exhibits maximum activity. The pH selected should beacidic toensurethatthechitin extractionyieldis sufficient. 0106. Accordingtooneembodiment,thereaction medium is ready for use when the temperature and pH conditions selected for the enzymatic hydrolysis reaction are stabilized. 0107 According to a first embodiment, the reaction medium comprises at least one acid. According to a second embodiment, the reaction medium further comprises a sol vent such as water or an aqueous solution. 0108. According to oneembodimentofthepresentinven tion, the acid used is preferably a dietary acid, preferably phosphoric acid or formic acid. 0109 Whentheacidused intheenzymatichydrolysisstep is a dietary acid, the products extracted by means of the methodaccordingtothepresentinventionoffertheadvantage of being suitable for easier use in the food and cosmetic SectOrS. 0110. According to one embodiment, the acid concentra tioninthereaction medium is from 0.1 to 6 mol-L', prefer ably from 0.8 to 2.8 mol-L, more preferably from 0.9 to 1 mol-L". 0111. According to one embodiment, the acid concentra tion in the reaction medium is adaptedto the mineral content oftheraw material usedsothatthepHofthereaction medium is acidic and remains constant throughout the enzymatic hydrolysis. 0112 0113. According to one embodiment, the enzyme used in the presentinvention is an activeenzyme in an acid medium, preferablyaproteasehavingabroadspectrum ofactivityinan acid medium, preferably pepsin ora stable acid protease. 0114. Accordingto oneembodiment,theenzymeconcen tration in the reaction medium is adapted to the protein con tentofthe raw material used. Accordingto one embodiment, theenzymeconcentration is0.1 to 75%, preferably 5 to30%, more preferably from approximately 23 to approximately 27% by mass in relation to the protein mass estimated in the raw material. According to one embodiment, the pH ofthe reac Enzyme Apr. 10, 2014 0115 Reaction Conditions 0116. According to one embodiment, the raw material is mixed with the reaction medium andthe resulting mixture is optionallyhomogenized by stirring for 0to 30 minutes, pref erably for3to 10 minutes, morepreferablyforapproximately 5 minutes. 0117. According to one embodiment, the ratio between the weight ofraw material prepared and the volume ofreac tion medium is 1:60 to 2:1, preferably 1:7 to 1:3, more pref erably equal to 1:5. 0118. According to one embodiment, the ratio between the weight ofraw material prepared and the volume ofreac tion medium is adapted to the size ofthe fragments of raw material prepared. In particular, account is taken ofthe fact that,whenthesizeofthefragmentsofraw material decreases, Solventabsorption increases andthat, consequently, it is nec essary to increase the Volume ofreaction medium. 0119) Adding raw material into the acid reaction medium may give rise to the formation offoam dueto theproduction ofcarbon dioxideduetothepresenceofcalcium carbonatein the exoskeleton ofcrustaceans and insects.Accordingto one embodiment, the vessel used for performing enzymatic hydrolysis has a suitable Volume for preventing the foam liable to form from overflowing. The riskoffoam production increases when the temperature of the acid before mixing increases. I0120 According to one embodiment, the temperature of thereaction mediumbeforeaddingtheraw materialis5to 65° C., preferably 20 to 30°C., more preferably approximately 25°C. In this embodiment, the temperature of the reaction medium is selectedin orderto beless than the temperatureat which the enzymatic hydrolysis is to be conducted, so as to limit foam formation when adding raw material. I0121 According to a first embodiment, the enzyme is added directly into the homogenized reaction medium optionally containing the raw material. 0122. According to a second embodiment, the enzyme is solubilized in water, or in a solution, preferably an aqueous Solution,andisaddedintothehomogenized reaction medium containing the raw material. I0123. According to one embodiment, the enzymatic hydrolysis reaction is performed under stirring so as to opti mize the contact between the raw material and the enzyme. 0.124. According to one embodiment, the initial pH and temperature conditions ofthe reaction medium are kept con stant throughout the enzymatic hydrolysis reaction. Accord ingtoafurtherembodiment,theinitialpHand/ortemperature conditions of the reaction medium are not kept constant throughout the enzymatic hydrolysis reaction. 0.125. According to one embodiment, the enzymatic hydrolysis reaction is performed in areactorequipped with a device for regulating the temperature. According to a first embodiment, said reactorisadouble-jacketreactorwherein a heat transfer fluid circulates, the temperature of said fluid beingpossibletocontrol.Accordingtoasecondembodiment, said reactor is equipped with a heating resistor, thetempera ture ofsaid resistor being suitable forbeing controlled. I0126. According to a first embodiment, the pH is stable throughoutthe enzymatic hydrolysis. According to a second embodiment, the pH is adjusted, during the enzymatic hydrolysis reaction, to the pKa value between the acid used and calcium carbonate by adding a concentrated acid solu tion, the acid being the same as that used in the reaction medium.
- 8. US 2014/0100361 A1 0127. According to one embodiment, the duration ofthe enzymatic hydrolysis is from 30 minutes to 24 hours,prefer ably from 1 hour to 12 hours, preferably from 3 hours to 8 hours, more preferably approximately 6 hours. 0128. According to one embodiment, the duration ofthe enzymatichydrolysis is adaptedto theactivity oftheenzyme usedforconductingtheenzymatic hydrolysis reaction, to the acid used and to the raw material. 0129. According to one embodiment, the duration ofthe enzymatic hydrolysis is adapted according to the features sought fortheendproducts, such as the degree ofpurity, the degree ofpolymerization and the degree ofacetylation. 0130. According to one embodiment, theenzymatic reac tion produces a reaction liquor comprising soluble and insoluble portions. 0131 Products Separation 0.132. According to one embodiment, the soluble and insolubleportions ofthe reaction liquorare separated by any suitable means known to those skilled in the art. 0.133 According to a first embodiment, the soluble and insoluble portions are separated by filtration. According to one embodiment, the filtration is performed by a filtration system preserving the integrity of the structures of the extracted compounds. According to a further embodiment, the filtration is performed by a membrane press filtration system. According to a further embodiment, the filtration is performed on a filter cloth, preferably on bolting cloth. 0134. Accordingtoa secondembodiment,the solubleand insoluble portions are separated by centrifugation. 0135 According to one embodiment, the insoluble por tion of the reaction liquor very predominantly contains chitins and the soluble portion contains various compounds Suchas lipids,pigments, Sugars, mineral salts, aminoacids or peptides. 0136. According to one embodiment, the insoluble por tion is rinsed using a solvent. According to a first embodi ment, the solvent is water or an aqueous Solution. This embodiment is preferred ifthechitins are subsequently used fordietary applications. Accordingto a secondembodiment, the insoluble portion is first rinsed with water or an aqueous Solution and then with a bleaching agent such as hydrogen peroxide, Sodium hypochlorite orpotassium perSulphateand is rinsedagain with wateroranaqueous solution.Thissecond embodiment is preferred ifbleachingofthechitins is sought. In this embodiment, the bleaching agents used are in accor dance with legislation. 0.137 Chitins are very hygroscopic substances, wherein the biological activity may be degraded by an increase in temperature. 0138 According to one embodiment, the filtered and rinsed insoluble portion is then dried for 8 to 16 hours, pref erably for approximately 12 hours, in an oven wherein the temperature is preferably less than 100° C., preferably between50and95°C.,morepreferablyapproximately90°C. 0.139. Accordingtooneembodiment,thefilteredinsoluble portion is neutralized with sodium hydroxide. According to oneembodiment, the insoluble portion is freeze-dried. 0140. According to one embodiment, the dried and/or freeze-dried insoluble portion is packaged in vessels such as glass orplastic bottles or vacuum pouches and storedprefer ably atambienttemperature in a dry place. Accordingto one particularembodiment, theinsolubleportion(chitin)isstored ata temperature less than ambienttemperature, preferably at Apr. 10, 2014 atemperaturefrom -30 to0°C., morepreferably from -20to -10°C., morepreferably at approximately -20°C. 0.141. Accordingtoafirstembodiment, thesolubleportion is centrifuged. According to a second embodiment, the soluble portion is dialysed and ultrafiltered. According to a third embodiment, the compounds from the neutralized soluble portion are extracted using organic solvents. The organic oraqueous solvents arethen evaporatedto beable to obtain the compounds ofinterest. 0142. The technique for processing the soluble phase is dependent on the nature ofthe compounds to be recovered. 0143. ExtractionYields 0144 Controlling the reaction medium (enzyme concen tration, pH and temperature) according to the raw material used makes itpossible to control the yieldand thebiochemi cal and physicochemical characteristics of the chitins obtained.Theoretically,byextendingthehydrolysistime,the degree ofpolymerization tends to be reduced. 0145 The mass extraction yield ofthe insoluble portions (Yd) is dependenton the nature ofthe raw material, acidand enzyme used and is calculated using the following formula: Yd%=100*(dried insoluble weight)/(dry raw material weight) 0146 According to one embodiment, the insoluble por tionpredominantly contains chitins andresidualproteinsand minerals not removed during the enzymatic hydrolysis reac tion. 0147 Applying conventional chemical extraction treat ment to the insoluble portions obtained using the method according to the present invention makes it possible to esti mate the residual impurity content in the insoluble portion. Indeed,this treatment is suitablefor removingthe majority of residual proteins and minerals. 0.148. The degree ofchitin purity (D purity) is estimated by means of gravimetry, by measuring the mass of the insoluble sample before and after treating the insoluble por tions with 1.25 mol-L' sodium hydroxide at 90° C. for 1 hour. As mentioned above, this treatment is suitable for removing residual proteins and minerals. The estimated degree ofpurity is calculated using the following formula: D-purity=100*(mass ofinsoluble portion aftertreat ment)/(mass ofinsoluble portion beforetreat ment) 0149 Accordingtooneembodiment,theestimateddegree of chitin purity (D'purity) is greater than 75%, preferably greater than 80%, more preferably greater than 85%, more preferably greater than 90%. 0150. According to one embodiment, the mass residual proteincontentinthedriedinsolubleportionisless than20%, preferably less than 15%, preferably less than 10%, more preferably less than 5%. 0151. According to oneembodiment, the massproportion ofproteins removed by the method according to the present invention is greater than 80%, preferably greater than 85%, more preferably greater than 90%, more preferably greater than 95%. 0152. According to one embodiment, the weight amount ofresidual mineral in the dried insoluble portion is less than 5%, preferably less than 3%, more preferably less than 1%. 0153. According to one embodiment, the weight amount ofminerals removed by the method according to the present invention is greater than 95%, preferably greater than 97%, more preferably greater than 99%.
- 9. US 2014/0100361 A1 0154 According to one embodiment, an additional bleachingoperation isperformedontheinsolubleportion, for removing pigments along with a portion ofthe residual pro teins and minerals. 0155 According to one embodiment, an additional deacetylation operation is performed on the insoluble por tion, for producing chitosan and removing a portion of the residual proteins. 0156 Features ofthe Chitins Extracted 0157 Accordingtooneembodiment,thechitins extracted using the method according to the present invention may be usedas is or converted into chitosan, chitin oligomers, chito san oligomers or optionally N-acetylated glucosamines. 0158. The method according to the present invention is Suitableforobtainingawiderangeofchitin qualityinrespect ofthedegreeofpurityandpolymerization.Theotherfeatures (pattern distribution, C, B and Y form) are dependent on the nature of the raw material and not on the features of the method according to the invention. 0159. Accordingtooneembodiment,thechitins extracted usingthe methodaccordingtothepresent inventionaresimi lar in form to the natural form ofchitin. In other words, the chitins extracted using the method according to the present invention are not denatured or only slightly denatured in relation to natural chitin. 0160 Accordingtooneembodiment,theestimateddegree ofpurity ofthe chitins extracted using the method according to the present invention is greater than 85%, preferably greater than 90%, more preferably greater than 95%. 0161 Thedegreeofpurity ofthechitinsobtainedusingthe method according to the present invention is sufficient to be able to convert said chitin in the form of chitosan, chitin oligomers, chitosan oligomers and glucosamines. 0162 According to one embodiment, the degree ofpoly merization ofthe chitins isestimatedby calculatingbasedon the average molecular mass ofsaid chitins. Accordingto one embodiment, the average molecular mass of the chitins is estimated by calculatingbased on the intrinsic viscosity. The intrinsic viscosity may be determined using the method described by Poirier et al. (Poirier, M. and Charlet, G., Car bohydrate Polymers, 2002, 50, 363-370). 0163 According to one embodiment, the degree ofpoly merization ofthe chitins extracted using the method accord ing to thepresentinventionis from 1.10 to 1.10, preferably from 1.10 to 1.107, more preferablyfrom 1.10 to 1.10. 0164. According to oneembodiment, the degreeofacety lation ofthe chitins extracted using the method according to the present invention is from 80% to 100%, preferably from 90% to 98%, more preferably from 95% to 97%. 0.165 According to one embodiment, the crystallinity index ofthe chitins extracted using the method according to the present invention is from 10% to 70%, preferably from 20% to 50%, more preferably from 30% to 40%. 0166 Soluble Compounds 0167 According to one embodiment, the soluble sub stances extracted using the method according to the present invention may be peptides, pigments, Sugars and mineral salts.Theuseofdietary acidinthis methodenables the useof these compounds in the food, dietetic and nutraceutical sec tOrS. 0168 The present invention thus offers the advantage of limiting the quantity ofwaste since all Substances other that chitin extracted using the method according to the invention can also be used or recovered. Apr. 10, 2014 0169. According to one embodiment, the pigments extractedusingthemethodaccordingtothepresentinvention are astaxanthin. BRIEF DESCRIPTION OF THE DRAWINGS (0170 FIG. 1 represents a scheme of the method for extracting chitin according to the invention. EXAMPLES 0171 The invention will be understood more clearly on readingthefollowing example, illustratingthepresentinven tion in a non-limiting fashion Example 1 Enzymatic Hydrolysis Using Pepsin in the Presence ofPhosphoricAcid 0172 Materials (0173 The raw material usedis the raw Panaeus vannamei prawn exoskeleton. The raw material is dried at 12° C. in ventilated air and ground to produce fragments less than 1 mm in size.The raw materialprepared is stored at -20°C. in a WaCl. 0.174. The reagent used to maintain theacidic pH is phos phoricacid.Theacidconcentration is calculatedaccordingto theinitial mineralcontentintheraw materialprepared. Foran initial mineral contentof25% in weightrelative to theweight ofdryraw material, a 0.94 mol-L' phosphoric acid solution is used to keep the pH ofthe reaction medium around 2. (0175. The acid protease used is pepsin (CAS 9001-75-6, supplier: Sigma, activity: 8112 U/mg). It is stored in powder form at +4°C. It is solubilised in distilled water for 15 min beforebeing introducedintothereaction medium. The quan tity ofenzymeadded in this example is equivalent to 25% of the estimated protein mass in the initial raw material. In this way, for a sample of 5 g ofraw material having a moisture content ofapproximately 15% andaprotein content ofclose to 40%, this isequivalent to8.5% ofenzyme in relation tothe raw material, i.e. a quantity ofpepsin of0.43g. (0176) 0177 5g ofraw material, prepared as describedabove, is weighed. The composition ofthe dry extract is determined according to the analytical methods described below. (0178 A 0.94mol-L' phosphoricacidsolution(25mL)is preheated to 30° C. and added to the raw material. The mix ture is stirred for 5 min so that it homogenises. The pH measured with a pH-meter should be stable and be between 1.9 and 2.1. 0179 Pepsin (0.43 g), previously solubilised in 1 mL of water,isadded to thereaction medium.Themixture isheated to 40°C. on a hotplate and incubated in an oven kept at 40° C.1° C. 0180. After6hoursofincubation,themixtureisfilteredon bolting cloth and rinsed with plenty of distilled water. The retentate is resuspended in distilled water, the mixture is stirred for 10 min beforebeing filtered and rinsedagain with water.Thesolidfractionobtainedis transferredintoacupand dried overnight at 90° C. in an oven. The mass ofdry extract obtained (m=1.29g) is suitable forcalculating theextraction yield, i.e. 30.26% w/w. Protocol
- 10. US 2014/0100361 A1 0181 Analyses 0182. The moisture content ofthe sample is measured by means ofgravimetry, by measuring the mass ofthe sample before and after being placed overnight at 105°C. 0183 The mineral content is determined by means of gravimetry, by measuring the mass ofthe sample before and after incineration at 600° C. for 6 hours. 0184 The protein content is estimated by means ofgas chromatographybyassayingthetotalaminoacids. Itcan also be measured by means ofa colorimetric assay (Lowry, BSA, Bradford orCoomassieblue)orby meansofa Kjeldahlassay. 0185. The chitin content can be measured by means of gravimetry, by measuring the mass ofthe sample before and after the following treatments: 0186 for the raw material: treatment for 60 minutes with 1NHClatambienttemperature, followedby 1.25N NaOH at 90° C. for 120 minutes and finally bleaching with 33% hydrogen peroxide and acetone; 0187 for the hydrolysis products, the treatment is lim ited to a treatment with 1.25 N NaOH at 90° C. for one hour. 0188 The molecular mass ofthe chitins is estimated by calculatingbased on the intrinsic viscosity. The intrinsic vis cosity can be determined using the method described by Poirier etal., which is based on the Mark-Houwink equation (Poirier, M. and Charlet, G., Carbohydrate Polymers, 2002, 50, 363-370). In this way, the intrinsic viscosity was deter minedby measuring the reduced viscosity using Solutions of variouschitinconcentrations inN,N-dimethylacetamidecon taining 5% LiCl. The apparatus used is an Ubbelohde capil lary viscometer. The viscometerconstant K is 0.3 cSt/s. The measurement Volume is 15 mL. 0189 Thedegreeofpolymerizationiscalculatedusingthe molecular mass ofthe chitins. 0190. The degree ofacetylation is estimated by means of protein liquid NMR, according to the method described by Einbu A. Varum K., 2008. Chitin (20 mg) is solubilized in 1 mLofDC1 (7.6N in DO, Euriso-top) with magnetic stirring atambient temperature for5hours.The "H NMR analysis is performed at 300 K using a Bruker ALS300 spectrometer (300 MHz, referenceTMSP 0.00ppm). The degreeofacety lation is then calculated basedon the intensity ofthecharac teristic proton NMR signals, according to the formula given by Einbu et al. 0191 The crystallinity index is determined by means of X-raydiffraction.Thediffractometerusedisa Bruker-axS D8 Discover (Karlsruhe, Germany). Radiation is produced in a coppertube(CuKC-1.5405A)and thebeamsproducedare recorded every 10 min. Using the spectra obtained, the method forcalculating the crystallinity index is based on the ratio between theareas ofthecrystalline Zones overthe total area (Osario-Madrazo A. David L., Trombotto S. Lucas J. M., Peniche-Covas C. and Domard A. Carbohydrate Poly mers, 2011, 83, 1730-1739). (0192 0193 After6hoursofenzymatichydrolysiswithpepsinin the presence of phosphoric acid, the extraction yield is 30.26+0.32% w/w. The composition of the dry extract obtainedcan becomparedto thatofthe fully dried raw mate rial orthe prepared raw material used in this example (table 1): Results and Discussion Apr. 10, 2014 TABLE 1 mois ture minerals proteins chitin lipids Sugars fully dry 8W material: % by mass O% 25% 40% 30% ND ND prepared 8W material: % by mass 14.55% 21.25% 34% 25.5% 3.5%. 1.2% dry extract: % by mass O% O.99 10.98 88.42 ND ND (+0.03%) (+1.01%) (+1.22%) per 100 g Og 0.30 g 3.32 g 26.76 g ND ND ofdry raw material ND: not determined 0194 In view ofthecomposition ofthe dry raw material, the quantities of minerals and proteins removed using the method are 98.5% and91.7%, respectively. 0.195 The residual mineral and protein contents (table 1) are those found in the unprocessed end product, without a bleaching step. Applying a bleaching agent or washing with Sodium hydroxide enhances the degree ofpurity. (0196. The degree ofacetylation measured by NMR is, in this example, in the region of95%. The molecular weight of this sample is in the region of 10 to 10 g/mol and the crystallinity index35%. These featuresaresimilarto those of native chitin. 0197) Theperformances ofthis examplecan be enhanced by increasing the quantity of pepsin used. In this way, the experiment was conducted with a pepsin concentration of 41% with respectto thequantity ofproteinspresentintheraw material, instead of 25% previously. The degree of chitin purityincreases (96.78%insteadof88.42%)as thedeprotein ization isenhanced(92.00% ofproteins removed)along with the demineralization (99.23% ofminerals removed). 1.A methodfortheenzymaticextractionofchitin,wherein said method is carried out in a single step, wherein chitin is obtainedby theenzymatichydrolyzis ofraw materialconsti tuted by animal biomass comprising chitin, said enzymatic hydrolyzis using an active enzyme in an acid medium. 2.A methodaccordingto claim 1, wherein said single step is an enzymatic hydrolysis fordeproteinizinganddemineral izing said raw material simultaneously. 3. A method according to claim 1, wherein said enzyme active in an acid medium is a protease having a broad spec trum of activity in an acid medium, preferably pepsin or a stable acid protease. 4. A method according to claim 1, wherein the enzyme concentration usedforhydrolysis is 0.1 to 75%,preferably 5 to 30%, more preferably from approximately 23 to approxi mately 27% in weight relative to the estimated weightofthe protein in the raw material. 5.A methodaccordingtoclaim 1,whereintheacidmedium is obtainedby means ofthe presence ofan acid, preferably a dietary acid, morepreferably phosphoric acid orformic acid. 6.Amethodaccordingto claim 1, whereinanimalbiomass comprising chitin comprises marine by-products, preferably
- 11. US 2014/0100361 A1 marine by-products obtained from crustaceans, preferably prawns, crabs orkrill, orfrom cephalopods,preferably squid or cuttlefish. 7. A method according to claim 1, wherein said animal biomass comprising chitin comprises insect by-products, preferably insect by-products obtained from beetles or hymenoptera. 8. A method according to claim 1, further comprising operations forwashing, drying and/orgrindingtheraw mate rial, preferably water washing, cold drying and/or grinding operations. 9.Amethodaccordingtoclaim 1, furthercomprising reac tion medium treatmentoperationsattheendoftheenzymatic hydrolyzis, said operations comprising operations for sepa rating the Solid and liquid phases, rinsing and/or drying the insoluble portion. 10. A method foroptimizingthe method fortheenzymatic extraction ofchitindescribedin claim 1, wherein said method comprises at least one ofthe following steps: a) selecting the pH of the acid medium in the range pHenzi-2, preferably pHenzit1.5, preferably pHenz+1, where pHenz is the pH at which the enzyme exhibits maximum activity, b)electingthetemperatureoftheacid medium intherange Tenzi-20° C., preferably Tenzi.15° C., preferably Tenzi-10°C.,whereTenzis thetemperatureatwhich the enzyme exhibits maximum activity, c) determining the mineral and protein content ofthe raw material, d) calculating the acid concentration to be used in the reactionmedium,accordingtothemineral contentofthe Apr. 10, 2014 raw material. Such thatthepH is maintainedthroughout the enzymatic hydrolysis atthe pH selected in step a), e) calculating the proportion of enzyme to be used with respect to the protein content ofthe raw material, f) determining the reaction time forobtaining chitin orthe chitin derivatives sought. 11. Chitin that can be obtained by means ofthe method according to claim 1. 12. Chitosan that can be obtained by deacetylating chitin according to claim 11. 13. Composition comprising chitin according to claim 11. 14. A pharmaceutical composition comprising chitin according to claim 11. 15.Acosmetic compositioncomprisingchitinaccordingto claim 11. 16.A medical device comprising chitin accordingto claim 11. 17. A food product, nutraceutical composition, dietetic composition,foodSupplementorfunctional food comprising chitin according to claim 11. 18.Acomposition comprisingchitinaccordingto claim 11 fortheuse thereofin watertreatment, filtrationand/ordepol lution. 19.Atexturingagentcomprising chitin accordingto claim 11. 20. A method according to claim 2, wherein said enzyme active in an acid medium is a protease having a broad spec trum of activity in an acid medium, preferably pepsin or a stable acid protease.