The need for Water Sanitaion
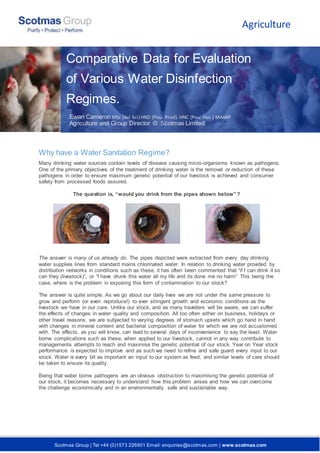
- 1. Scotmas Group | Tel +44 (0)1573 226901 Email: enquiries@scotmas.com | www.scotmas.com Why have a Water Sanitation Regime? Many drinking water sources contain levels of disease causing micro-organisms known as pathogens. One of the primary objectives of the treatment of drinking water is the removal or reduction of these pathogens in order to ensure maximum genetic potential of our livestock is achieved and consumer safety from processed foods assured. The question is, “would you drink from the pipes shown below”? The answer is many of us already do. The pipes depicted were extracted from every day drinking water supplies lines from standard mains chlorinated water. In relation to drinking water provided by distribution networks in conditions such as these, it has often been commented that “if I can drink it so can they (livestock)”, or “I have drunk this water all my life and its done me no harm” This being the case, where is the problem in exposing this form of contamination to our stock? The answer is quite simple. As we go about our daily lives we are not under the same pressure to grow and perform (or even reproduce!) to ever stringent growth and economic conditions as the livestock we have in our care. Unlike our stock, and as many travellers will be aware, we can suffer the effects of changes in water quality and composition. All too often either on business, holidays or other travel reasons, we are subjected to varying degrees of stomach upsets which go hand in hand with changes in mineral content and bacterial composition of water for which we are not accustomed with. The effects, as you will know, can lead to several days of inconvenience to say the least. Water borne complications such as these, when applied to our livestock, cannot in any way contribute to managements attempts to reach and maximise the genetic potential of our stock. Year on Year stock performance is expected to improve and as such we need to refine and safe guard every input to our stock. Water is every bit as important an input to our system as feed, and similar levels of care should be taken to ensure its quality. Being that water borne pathogens are an obvious obstruction to maximising the genetic potential of our stock, it becomes necessary to understand how this problem arises and how we can overcome the challenge economically and in an environmentally safe and sustainable way. Agriculture Comparative Data for Evaluation of Various Water Disinfection Regimes. And Other Ewan Cameron MSc (Avi Sci) HND (Pou. Prod). HNC (Pou. Hus.) MAAAP Agriculture and Group Director © Scotmas Limited
- 2. Biofilm Basics Contrary to common belief, most bacteria are not “free living” – instead, they live in a self- sustaining community where they cooperate with other bacteria and are largely protected from outside influences. This is properly known as a “Biofilm” although most people would recognise it as “slime”. This makes up a large proportion of the brown deposits you see on the pipework above. Among many other bacteria, Biofilm harbours the dangerous pathogens responsible for poor growth reduced liveability and poor food conversation to mention but a few. Biofilm removal is the most important part of any drinking water treatment system. If the sanitation product used does not have the ability to remove biofilm it is impossible to have a safe pathogen free environment. Many products, even down to the level of citrus juices, have the ability to kill free living bacteria, but only the best have the ability to penetrate and destroy biofilm In the early days since recognition of this problem in the Poultry industry, application of basic industrial water treatment products such as occasional addition of chlorine tablets have shown some benefit over doing nothing, however due to their inability to tackle the complex problem of biofilm forming throughout the water pipe-work over a long growth cycle, these approaches have been found to be not necessarily protective or economic. Since the early 90’s vast improvements have been made in biocide dosing technology and chemical monitoring equipment, which when combined with new microbiological analysis techniques have proven to secure the viability of treatment programs. Currently there are a number of “new generation” water treatment approaches being used, ranging from professional ozone and chlorine dioxide dosing units, through to the addition of Peracetic acid, pH adjusters and even so called “organic bio-flavonoids. Biofilms form when bacteria adhere to surfaces in aqueous environments and begin to excrete a slimy, glue-like substance that can anchor them to a variety of materials including metals, plastics, soil particles, medical implant materials and, most significantly, human or animal tissue. If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion molecules, proteins on their surfaces that bind other cells in a process called cell adhesion. These bacterial pioneers facilitate the arrival of other pathogens by providing more diverse adhesion sites. They also begin to build the matrix that holds the biofilm together. If there are species that are unable to attach to a surface on their own, they are often able to anchor themselves to the matrix or directly to earlier colonists. The first substances associated with the surface are not bacteria but trace organics. Almost immediately after the clean pipe surface comes into contact with water, an organic layer deposits on the water/solid interface (Mittleman 1985). These organics are said to form a conditioning layer which neutralises excessive surface charge and surface free energy which may prevent a bacterium cell from approaching near enough to initiate attachment. In addition, the adsorbed organic molecules often serve as a nutrient source to bacteria. In a pipe of flowing water, some of the planktonic (free-floating) bacteria will approach the pipe wall and become entrained within the boundary layer, the quiescent zone at the pipe wall where flow velocity falls to zero.
- 3. Some of these cells will strike and adsorb to the surface for some finite time and then desorb. This is called reversible adsorption. This initial attachment is based on electrostatic attraction and physical forces, not chemical attachments. Some of the reversibly adsorbed cells begin to make preparations for a lengthy stay by forming structures which may permanently adhere the cell to the surface. These cells become irreversibly adsorbed. Biofilm bacteria excrete extracellular polymeric substances,or sticky polymers, which hold the biofilm together and cement it to the pipe wall. In addition, these polymer strands trap scarce nutrients and protect bacteria from most biocides. According to Mittleman (1985), “attachment is mediated by extracellular polymers that extend outward from the bacterial cell wall (much like the structure of a spider’s web). This polymeric material, or glycocalyx, consists of charged and neutral polysaccharide groups that not only facilitate attachment but also act as an ion-exchange system for trapping and concentrating trace nutrients from the overlying water. The glycocalyx also acts as a protective coating for the attached cells which mitigates the effects of biocides and other toxic substances.” As nutrients accumulate, the pioneer cells proceed to reproduce. The daughter cells then produce their own glycocalyx, greatly increasing the volume of ion exchange surface. Pretty soon a thriving colony of bacteria is established. (Mayette 1992). In a mature biofilm, more of the volume is occupied by the loosely organised glycocalyx matrix (75-95%) than by bacterial cells (5-25%) (Geesey 1994). Because the glycocalyx matrix holds a lot of water, a biofilm- covered surface is gelatinous and slippery. The mature, fully functioning biofilm is like a living tissue on the pipe surface. It is a complex, metabolically co-operative community made up from different species each living in a customised micro-niche. Biofilms are even considered to have primitive circulatory systems. Mature biofilms are imaginatively described as follows in an article called Slime City (Coghlan 1996): “Different species live cheek-by-jowl in slime cities, helping each other to exploit food supplies and to resist antibiotics through neighbourly interactions. Toxic waste produced by one species might be hungrily devoured by its neighbour. And by pooling their biochemical resources to build a communal slime city, several species of bacteria, each armed with different enzymes, can break down food supplies that no single species could digest alone. “The biofilms are permeated at all levels by a network of channels through which water, bacterial garbage, nutrients, enzymes, metabolites and oxygen travel to and fro. Gradients of chemicals and ions between micro-zones provide the power to shunt the substances around the biofilm.” A biofilm can spread at its own rate by ordinary cell division and it will also periodically release new “pioneer” cells (commonly Pseudonomas aeruginosa) to colonise downstream sections of pipework. As the film grows to a thickness that allows it to extend through the boundary layer into zones of greater velocity and more turbulent flow, some cells will be sloughed off. According to Mayette (1992): “These later pioneer cells have a somewhat easier time of it than their upstream predecessors since the parent film will release wastes into the stream which may serve as either an initial organic coating for uncolonised pipe sections downstream or as nutrient substances for other cell types.”
- 4. The Microbiology of Biofilms (Summary) Bacteria are initially attracted to pipe surfaces for lots of reasons. They may be attracted to the positive charge on some inorganic surface because they often have a negatively charged outer envelope themselves, or they may arrive and settle out due to gravity or water flows. There is evidence that biofilm formation is much more than simple random physical force. Many surfaces attract and concentrate nutrients, and many bacteria have the skill to detect and follow such high concentrations (an ability called chemotaxis). Some cells produce lots of polysaccharides which act as mucus layers, gaining a foothold and gluing others to the selected host surface. These are called the primary colonisers. This external slime gives a helping hand to other passing bacteria who add another tier of inhabitants called the secondary colonisers who live and feed on the waste produced by the first colonisers. Before long a thriving complex microbial community has been established inside the polysaccharide slime and this is called the biofilm. Protection from antibiotics and biocides. Much higher doses of antibiotics & biocides are required to kill bacteria in biofilms compared with their freewheeling relatives. At first, it was thought that the biofilm provided a complete physical barrier to efforts at destruction but there is now evidence that the very nature of the colonies themselves provides protection. By growing in colonies, the late arrivals protect the inner cells from penetration, leaving the latter free to grow and multiply. Concentration of nutrients As negative charges are often associated with the biofilm matrix, many nutrients are drawn to settle and nutrients with negative charges can exchange with ions on the surface. This activity provides an attractive source of nourishment compared to the surrounding water. Community feeling No bacterium likes to be alone for long and nearly all live with other micro-organisms for energy, carbon and other nutrients. We see a classic example in the degradation of cellulose; celluolytic microbes break the cellulose into sugar monomers which fermenting bacteria can use, giving off smaller organic acids, carbon-dioxide and hydrogen gas, which methanogens or sulfate reducers use for their carbon and energy. Finally, genetic material can be easily exchanged within the close confines of the biofilm. This increases the potential for the successful emergence of better adapted and stronger strains of bacteria. According to Mittleman (1985), the development of a mature biofilm may take several hours or several weeks, depending on the water delivery system. Pseudomonas aeruginosa is a common pioneer bacterium and is used in a lot of biofilm research. In one experiment (Vanhaecke 1990) researchers found that Pseudomonas cells adhere to stainless steel, even to electro polished surfaces, within 30 seconds of exposure.
- 5. The life Cycle of Biofilms Each number corresponds with a stage in biofilm development. The photomicrographs are of an actual Pseudomonas aeruginosa biofilm The far right photograph depicts a biofilm far from being microscopic! But never the less this advanced formation is all too common. Chlorine Dioxide and Chlorine Chlorine Disinfection of Water Chlorine disinfection can be achieved either by the use of chlorine gas or by using sodium or calcium hypochlorite. When free chlorine gas is dissolved in water, a mixture of hydrochloric and hypochlorous acids is formed which will react with water by dissociation to an extent determined by pH. At pH 7 approximately 80% remains effective as hypochlorous acid. As pH increases this figure falls dramatically so that at pH 8 only around 20% remains active. Optimum use of chlorine requires pH control. When sodium or calcium hydroxide which increases the pH thereby limiting the efficacy of the active acids. Chlorine as hypochlorous acid readily reacts with both organic and non-organic material such as sulphides and ammonia. Initially the chlorine will be used up in these reactions and testing may show that none remains as a residual, in the water. As further hypochlorous acid is added ammonia and some organics will react to form to produce chloramines and chloro-organic compounds resulting in taste and odour problems. Additional hypochlorous acid will be used in oxidising the compounds just formed and only after that has happened will there be an available residual in the water. Chlorine will start to dissociate or "gas off" from water at about 40oC.
- 6. Although chlorine will oxidise as a tertiary reaction, its primary reaction is chlorination and many of the chloro-organics formed will not be re-oxidised. These residuals include the group trihalomethanes, which are known carcinogens and difficult and costly to remove, especially once they have contaminated the ground water. When sodium or calcium hypochlorite are used, the chemical reactions involved also produce sodium and calcium free chlorine gas is toxic and corrosive when in solution. Biocidal effectiveness is limited to simpler organisms and viruses in operating conditions of pH6.5 - 8 and below 40oC. Chlorine has limited effectiveness against biofouling and will not kill cysts and protozoa. Chlorine Dioxide Disinfection of Water Chlorine dioxide is a powerful oxidising biocide and has been successfully used as a water treatment disinfectant for several decades in many countries. Chlorine dioxide is a relatively stable radical molecule. It is highly soluble in water, has a boiling point of 110C, absorbs light and breaks down into ClO3- and Cl-. Because of its oxidising properties chlorine dioxide acts on Fe2, Mn2+ and NO2- but does not act on Cl, NH4+ and Br- when not exposed to light. These ions are generally part of the chemical composition of natural water. Because of its radical structure, chlorine dioxide has a particular reactivity - totally different from that of chlorine or ozone. The latter behave as electron acceptors or are electrophilic, while chlorine dioxide has a free electron for a homopolar bond based on one of its oxygens. The electrophilic nature of chlorine or hypochloric acid can lead, through reaction of addition or substitution, to the formation of organic species while the radical reactivity of chlorine dioxide mainly results in oxycarbonyls. Generally chlorine dioxide rapidly oxidises phenol type compounds, secondary and tertiary amines, organic sulphides and certain hydrocarbon polycylic aromatics such as benzopyrene, anthracene and benzoathracene. The reaction is slow or non-existent on double carbon bonds, aromatic cores, quinionic and carboxylic structures and primary amines and urea. The reduction of bad tastes and odour with ClO2 is the result of the elimination of algae and on the negligible formation of organo-chlorinated derivatives. When chlorine dioxide replaces chlorine in a system it may take up to 15 days for the benefits of the change to become apparent. Changes should be made gradually to avoid problems of a sudden release of slime into the system.
- 7. Chlorine by Category Chlorine Dioxide by Category Dose Rates and Efficiency The dose rate, or CT time, is a measure of the disinfectant contact time. It is the mathematical product of C (the concentration of disinfectant in mg/l) and the contact time T measured in minutes to achieve 99% deactivation. Table 1 shows a variety of microorganisms and their corresponding CT values for chlorine at pH 6 – 7. This is also an indication as to the “speed of kill” of particular pathogenic material – the lower the CT value, the less time it takes to destroy the bacterium. Chlorine Table 1 - CT ValuesforChlorine (Cl2) Microorganism Disinfectant - Free Cl 2 E.Coli 0.034 - 0.05 Polio 1 1.1 - 2.5 Rotavirus 0.01 - 0.05 Giardia lamblia cysts 47 - 150 Giardia muris cysts 30 - 630 Cryptosporidium parvum 7200 Table 2 demonstratesthe CTValuesagainstthose seeninTables1for free chlorine
- 8. Chlorine Dioxide Table 2 - CT Valuesfor ClO2 It can be seenthatthese valuesare muchlowerthantheirchlorine counterpartsandissubstantial proof that ClO2 ismore effective overashortertime span. Microorganism Disinfectant - Free ClO 2 E.Coli 0.4 - 0.75 Polio 1 0.2 - 6.7 Rotavirus 0.2 - 2.1 Giardia lamblia cysts 26 Giardia muris cysts 7.2 - 18.5 Cryptosporidium parvum 78 Hydrogen Peroxide and Peracetic acid Compounds Hydrogen peroxide, as a disinfectant, required a concentration of 258,000 mg/l and a detention time of 17 minutes to inactivate S. Aureus, spores of Bacillus, and Clostridium. Inactivation of 99% of poliovirus was obtained over a 6hr contact time. The peroxide concentration was 3,000 mg/l. From this data, hydrogen peroxide was clearly not a suitable drinking water disinfectant. “Committeeon Products & Processes” The Use of Disinfectants Containing Silver Salts in Public Water Supplies Summary On several occasions over the last few years the Committee on Products and Processes for Usein Public Water Supply (CPP) has been asked to consider the use of disinfectants containing silver salts, in the continuous disinfection of drinking water supplies. Recently the CPP has been asked again to consider an application for the use of a productbased on hydrogen peroxide containing a silver salt as a disinfectant for the continuous treatment of water for public supply. After consideration of the data provided, ” the CPP concluded that productscontaining silver saltscould not be recommended for approval for continuoususe as disinfectantsfor water for public supply.”
- 9. IndependentBiocidal Comparative testing Comparative testing of Chlorine Dioxide and Peracetic Acid were conducted as described in (Tanner R.S. 1989 Comparative testing and evaluation of hard-surface disinfectants. J. Indust.Microbiol 4:145-154) with the following exceptions; a. Cultures of a strain of Pediococcus and a mixed culture of Lactobacillus sp. were obtained from a brewing company. b. 10 mM potassium phosphate was included in the medium to stimulate the growth and viability of the lactic acid bacteria which are dependent on substrate 1evel phosphorylation for metabolism and growth). c. Test cultures were incubated for 48 hr at 30oc. enumeration plates ware incubated for 72 hr. d. Sodium thiosulphate (5,000 ppm) was used to neutralize each of the oxidising biocides tested. e. Tile tests containing Peracetic Acid (100, 200, and 500 ppm) also contained hydrogen peroxide (620, 1,200, and 3,100 ppm, respectively). Level of Disinfectants Required to Achieve A 99.999% Kill Disinfectant Microorganism Pseudomonas aeruginosa Staphylococcus aureus Saccharomyces cerevisiae ChloroxTM 1000 1000 1000 Sodium Chlorite 820 820 1000 Chlorine Dioxide 48 93 95 Iodophor 440 440 450 Peroxide 36000 68000 270000 Gluteraldehyde-Phenol 2300 1200 620 Acid Gluteraldehyde 6600 2200 18000 Quaternary 580 140 740 Acidifi Quaternary 150 1200 300 Phenolic 1500 380 190 Published by ALLTECH Biotechnology Centre, USA
- 10. Condition Ppm Variable Cells/ml (60 second exposure) Pediococcus Lactobacillus Control 2.5 x 106 1.6 x 106 Chlorine Dioxide 20 <2 x 100 <2 x 100 50 <2 x 100 <2 x 100 100 <2 x 100 <2 x 100 Peracetic Acid 100 >105 >105 200 8.5 x 102 >105 500 <2 x 100 7.3 x 105 The results indicate that 20 ppm Chlorine Dioxide could reduce viable counts of lactobacillus bacteria at least 99 999% in a 60 second speed-of-kill assay. The results suggest that about 1000ppm of Peracetic Acid would be required to achieve a similar reduction in viability, using this test. - Dr R.S. Tanner, Sept 14 1991 Product Stability System where Hydrogen Peroxide has to be diluted and dosed via appliances such as a dosatron will encounter drastic drops in efficiency as demonstrated in the chart below. Hydrogen peroxide is not stable and has an extremely short half life (Evaluation of Different Hydrogen Peroxide Products for Maintaining Adequate Sanitizing Residual in Water.Tyler Clark, Brookee Dean and Susan Watkins University of Arkansas, Division of Agriculture) Scotmas provide a fully automatic package of equipment and water treatment chemicals, with no pre-dilution required. Premixing Peroxide involves exposure to hazardous chemicals and has led numerous incidents of skin burns. Hydrogen Peroxide is classified as a Tier 2 Explosive Precursor under European Regulations, and sites using the product in concentrations above 15% require appropriate regulatory licenses and notifications to be made in various countries. (Standard products are sold as 35% solutions).
- 11. Chlorine Dioxide and Ozone, UV Chlorine Dioxide is a versatile oxidant /disinfectant with CT values second only to ozone in biocidal efficacy, but without the high capital expenditures and associated by- products. As neither UV or Ozone are regarded as having any meaning full residuals there use in agriculture and livestock drinking systems is considered non-viable. A brief note on the comparative qualities is however detailed below Disinfectant Residual Maintenance Information on By-products Colour Removal Removal of Common Odors Chlorine Good Adequate Good Moderate Chloramines Good Limited Unacceptable Poor Chlorine Dioxide Excellent Good Good Excellent Ozone Unacceptable Limited Excellent Excellent Ultraviolet Unacceptable Nil N/A N/A Comparative Oxidation Strengths • Because chlorine and other oxidants reacts with more compounds (due to higher oxidation strength), less is available to react with bacteria making them significantly poor biocides. • This means that their dose rates must be higher to achieve if at all possible similar kill rates • This causes problems of water palatability (The typical concentration of chlorine in a swimming pool is 2-3ppm – would you drink that?) • Higher concentrations make corrosion problems even worse Oxidant Oxidation Strength Oxidation Capacity Ozone (O3) 2.07 2 e- Hydrogen Peroxide (H2O2) 1.78 2 e- Hypochlorous Acid (HOCl) 1.49 2 e- Hypobromous Acid (HOBr) 1.33 2 e- Chlorine Dioxide (ClO2) 0.95 5 e-
