Lecture 18.2a- Equilibrium
•
3 gefällt mir•1,123 views
Section 18.2 (part A) lecture for Honors & Prep Chemistry
Melden
Teilen
Melden
Teilen
Downloaden Sie, um offline zu lesen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Chem 2 - Chemical Equilibrium VI: Heterogeneous Equilibria

Chem 2 - Chemical Equilibrium VI: Heterogeneous Equilibria
Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...

Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...
Andere mochten auch
Andere mochten auch (11)
Chem 2 - Chemical Kinetics VIII: The Arrhenius Equation, Activation Energy, a...

Chem 2 - Chemical Kinetics VIII: The Arrhenius Equation, Activation Energy, a...
Ähnlich wie Lecture 18.2a- Equilibrium
Ähnlich wie Lecture 18.2a- Equilibrium (20)
Core & Extension - Chemical Rxns - Reversible Rxns I.pptx

Core & Extension - Chemical Rxns - Reversible Rxns I.pptx
Chapter 6_Chemical-Equilibrium_Le Chateliers Principle-1.pdf

Chapter 6_Chemical-Equilibrium_Le Chateliers Principle-1.pdf
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 1 WRI...

CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 1 WRI...
Mehr von Mary Beth Smith
Mehr von Mary Beth Smith (20)
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Proteins (part a)
Kürzlich hochgeladen
This PowerPoint presentation, titled "Research Methods in Psychology for Cambridge AS Level Students," provides a comprehensive overview of essential research methodologies in psychology. It covers fundamental concepts such as experimental, correlational, and observational methods, highlighting their advantages and limitations. The presentation delves into the design of experiments, including independent and dependent variables, control groups, and random assignment. It also addresses ethical considerations, data collection techniques, and statistical analysis. Emphasizing practical application, the presentation includes examples of classic psychological studies and offers tips for designing and conducting research projects. It concludes with a discussion on interpreting results and the importance of critical evaluation, preparing students for both theoretical understanding and practical application in their AS Level psychology coursework.Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...

Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...Abhinav Gaur Kaptaan
Kürzlich hochgeladen (20)
Post Exam Fun(da) Intra UEM General Quiz 2024 - Prelims q&a.pdf

Post Exam Fun(da) Intra UEM General Quiz 2024 - Prelims q&a.pdf
Application of Matrices in real life. Presentation on application of matrices

Application of Matrices in real life. Presentation on application of matrices
Removal Strategy _ FEFO _ Working with Perishable Products in Odoo 17

Removal Strategy _ FEFO _ Working with Perishable Products in Odoo 17
Danh sách HSG Bộ môn cấp trường - Cấp THPT.pdf

Danh sách HSG Bộ môn cấp trường - Cấp THPT.pdf
Basic Civil Engg Notes_Chapter-6_Environment Pollution & Engineering

Basic Civil Engg Notes_Chapter-6_Environment Pollution & Engineering
Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...

Research Methods in Psychology | Cambridge AS Level | Cambridge Assessment In...
Pragya Champions Chalice 2024 Prelims & Finals Q/A set, General Quiz

Pragya Champions Chalice 2024 Prelims & Finals Q/A set, General Quiz
Features of Video Calls in the Discuss Module in Odoo 17

Features of Video Calls in the Discuss Module in Odoo 17
Lecture 18.2a- Equilibrium
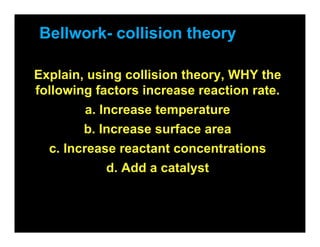
- 1. Bellwork- collision theory Explain, using collision theory, WHY the following factors increase reaction rate. a. Increase temperature b. Increase surface area c. Increase reactant concentrations d. Add a catalyst
- 2. At chemical equilibrium, no change occurs in the amounts of the products and reactants. At equilibrium the system is stable H2O(s) ⇌ H2O(l) equilibrium at 0°C Means the process is at equilibrium
- 3. A reversible reaction is one in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously. forward 2SO2 + O2 ⇌ 2SO3 reverse 2SO3 ⇌ 2SO2 + O2
- 4. 2SO2 + O2 ⇌ 2SO3 There are 6 mol SO2 and 3 mol O2 in a closed container As the reaction progresses, reactants form products. The forward reaction rate SLOWS as reactant concentration decreases. The reverse reaction rate INCREASES as product concentration increases.
- 5. 2SO2 + O2 ⇌ 2SO3
- 6. 2SO2 + O2 ⇌ 2SO3
- 7. When the rates of the forward and reverse reactions are equal, the reaction has reached a state of balance called chemical equilibrium.
- 8. At equilibrium the concentrations of all “species” are constant. Equilibrium position = the specific concentrations of all species at equilibrium, which can be calculated for any reaction.
- 9. SO2 and O2 SO3 react to give decomposes SO3 to SO2 and O2 At equilibrium, all three types of molecules are present.
- 10. If the rate of the shoppers going up the escalator is equal to the rate of the shoppers going down, then the number of shoppers on each floor remains constant, and there is an equilibrium.
- 11. In order to reach equilibrium you need A closed container Stable temperature Low activation energies
- 12. The equilibrium constant (Keq) is a ratio of product concentrations to reactant concentrations at equilibrium. For aA + bB cC + dD Coefficients Keq = [C]c[D]d become [A]a [B]b exponents!
- 13. A value of Keq greater than 1 means that products are favored over reactants; A value of Keq less than 1 means that reactants are favored over products. products =K reactants
- 14. 18.2 Section Quiz. 1. In a reaction at equilibrium, reactants and products a) decrease in concentration. b) form at equal rates. c) have equal concentrations. d) have stopped reacting.