Plant metabolism and redox agents plant biochemistry ii
- 1. PLANT BIOCHEMISTRY II Role of Redox agents and plant metabolism
- 2. Metabolism •"The sum of total chemical activities of all cells ." • Plant metabolism is the set of complex of p hysical and chemical events of photosynthesis, r espiration and the synthesis and degradation of organic compounds.
- 3. • Photosynthesis produces the substrates fo r respiration and the starting organic co mpounds used as building blocks for sub sequent biosynthesis of nucleic acids, a mino acids, and proteins, carbohydrates and organic acids, lipids, and natural pro ducts.
- 6. Types of metabolic pathway 1.Catabolic pathway • Involved in the breakdown of large molecule. • Commonly involving oxidation reactions. • Theses pathways are exothermic(release energy). • e.g; respiration.
- 7. 2 . Anabolic pathway Involved in the synthesis of large and more complex compounds from small molecules. Theses pathways are endothermic. E.g photosynthesis.
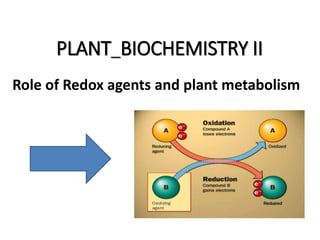
- 8. Biological oxidation reactions Biological oxidation is that which occur in biological system to produce energy. Oxidarion can occur by : Addition of oxygen (less common). Removal of hydrogen ( common). Removal of electron (most common). Electrons are not stable in free state.so, there removal from substance (oxidation) must be acco mpained by another substance (reduction). Hence the reaction is called oxidation -reduction reaction or redox reaction. And the invovled enzymes are called oxido- reductases enzymes.
- 10. Transfer of electrons Transfer of electrons can take place by 4 different ways 1. Directly as electrons 2. As hydrogen atom (H) 3. AS an hydride ion (:H-) electron donor which has 2 electron ..which occur in case of NAD+ linked dehydrogenases 4 . Though as direct combination with oxygen.
- 11. Enzymes invovled in redox Reaction are called as oxidoreductases which include : Oxidases Dehydrogenases Hydroperoxidases Oxygenases. Oxidases and dehydrogenases are involved in respi ration, Hydroperoxidases neutralizes free radicals & Oxygenases are involved in biotransformation re actions. Oxidation is often synonymous with dehydrogenati on and many enzymes catalyze oxidation reactions are called dehydrogenases
- 12. Redox potential • It is the affinity of a substance to accept electrons i.e; it is the potential of a substance to become reduced. • hydrogen has lowest redox potential (-0.42 volt) while oxygen has the highest redox potential (+0.82 volt). • The redox potential of all substances lie between the hydrogen and oxygen. • Electrons are transferred from substances with low redox potential to substance with higher redox potential .This transfer of electrons is an energy yielding process and the amount of energy liberated depends upon the redox potential difference between the electron donor and acceptor.
- 14. Biological oxidation reactions •Reducing equivalent (proton and electron) derived from oxidations of substrate s (cellular respiration) are transported to the electron transporters/ carriers. Electron carries Hydro soluble coenzymes Nicotina amide coenzymes Flavin coenzymes lipid soluble coenzymes( quinones & plasto quinones) Fe-S proteins Cytochromes.
- 15. Electron carries Nicotinamide coenzymes - Nicotinamide adenine dinucleotide( NAD+). - Nicotinamide adenine dinucleotide p hosphate(NADP+) • These are the coenzymes of enzym e dehydrogenases • NAD+ and NADP+ are differ only in the presence or absence of phosph ate group on C-2 of moiety. • NAD+ and NADP+ can be reduced t o generate NADH and NADPH resp ectively.
- 16. Nicotinamide coenzyme...... • They are derived from niacin (nicotin acid)(B3) _ to provide nicotin amide portion. • They transfer hydride (:H-) to and from the reactants. • The reduced coenzymes NADH and NADPH then serves a s reductants in other reactions. • In Glycolysis and citric acid cycle NAD+ oxidizes glucose a nd releases energy which is then transfers to NAD+ by red uction to NADH. • NAD+ is act as coenzymes for various enzymes like Pyruvate dehydrogenases ,glyceraldehyde phosphate Dehydrogenase(in glycolysis) and 4 dehydrogenations In citric cycle..
- 18. Oxidative decarboxylation (Pyruvate dehydrogenases) • In the oxidation process electron along with protons are transferred from pyruvate to NAD+. • Energy released by oxidation of pyruvate is transferred to NADH.
- 19. (Glyceraldehyde-3-phosphate dehydrogenases) • Redox reaction. • Glyceraldehyde-3-phosphate is oxidized . • NAD+ is reduced to NADH. • This occur when a hydride (:H-) is transferred from G-3- P 's carbonyl carbon to NAD+. • This reduction requires energy which comes from G-3-P.
- 20. 4 dehydrogenations in citric cycle.
- 22. • They are forms of riboflavin (vita.B2). • They contain ribitol and a flavin (isoalloxazine ring). • The ribitol is linked to phoshate in FMN. • FAD is formed from FMN by transfer of an AMP from ATP. • 2 coenzymes : flavin mono nucleotide (FMN). And flavin adenine dinucleotide(FAD). • The isoalloxazine ring is reduced by accepting one or two hydrogen atoms from reduced substrate. • Flavin coenzymes are very tightly bound or covalently bound to flavoprotein. • The redox potential of isoalloxazine ring depend upon specific flavoprotein.
- 25. Biological functions and importance FAD and FMN The enzymes that use flavin coenzymes are called Flavoproteins . FAD has more positive reduction potential than NAD+. Is very strong oxidizing agent.. FAD act as coenzyme for various enzymes like alpha ketoglutarate dehydrogenase , succinate dehydrogenase, lipid metabolites, Xanthanin dehydrogenase and acyl CoA dehydrogenases. • It is involved in electron transport chain and play role in production of ATP. • The reduced coenzyme FADH2 contributes to oxidative phosphorylation in the mitochondria.FADH2 is re-oxidized to FAD which make it possible to produce 1.5 equivalent of ATP.
- 26. • Beta oxidation of fatty acids Redox flavoproteins that not covalently bind to FAD like Acetyl-coA dehydrogenases which are involved in beta ox idation of fatty • Mitochondrial Glycerol 3- dehydrogenase It catalyses the conversion of glycerol 3 phosphate to DH AP in mitochondria. It is essential for carrying reducing equivalents from cytosol to mitochondria.
- 27. Alpha- ketoglutarate dehydrogenase complex • It catalyzes the oxidative decarboxylation of alpha -ketoglutarate to succinyle CoA . • FAD is present in dihydrolipoyldehydrogenase of alpha-ketuglutarate dehydrogenase complex.
- 28. Cytochromes. • Cytochromes are proteins. • Cytochromes are electron carriers containing heme prosthetic group s which functions as one electron carriers. • The heme iron is involved in one electron transfers involving the Fe2+ and Fe3+ oxidation states. • Heme in the 3 classes of cytochromes (a ,b ,c)differ in the substituents on the porphyrin ring. • Some cytochromes (b, c1, a, a3)are part of large integral membrane protein complexes. • Cytochrome c is a small water soluble protein.
- 30. Fe-s(iron sulfur proteins) • These are non - heme iron-sulfur protein in which iron is tetrahedrally coordinated by four cysteine. • These are participate in one electron transfer involving the Fe2+and Fe3+. • Involved in electron transport chain of respiration.
- 31. Ubiquinones • Co enzyme Q (CoQ ,Q or ubiquinone) is lipid soluble . • It is soluble electron carrier in the hydrophobic bilipid layer of inner mitochondrial membrane . • The only electron carrier not bound to a protein. • It can accept 1 or 2 e-.
- 32. Respiratory chain Electron transport chain (ETC) • The ETC is a series of membrane bound electron carriers arranged in stepwise of increasing redox potential . • it collects reducing equivalents( H-atoms and electron). • It is also known as redox chain or respiratory chain. • Embedded in the inner mitochondrial membrane • Electrons from NADH and FADHH2 are transferred to complexes of the ETC. • Each complex transfers the electron to the next complex in chain.
- 34. Complexes of ETC. • Complex l NADH dehydrogenase ( NADH -Ubiquinone oxido- reductase) It is flavoprotein that contain FMN as well as Fes protein as co enzyme transfers hydrogen from NADH +H to ubiquinone. • Complex ll: Succinate dehydrogenases(succinate -ubiquinine oxidoreductase) It is flavoprotein that contain FAD as well as Fe S protein as coenzyme it transfers H-atoms from succinate to ubiquinones. • Complexlll : ubiquinol dehydrogenase ( ubiquinol cytochrome oxidoreductases ) It transfers electron from ubiquinol to cytochrome using cytochrome b and cytochrome c1 as coenzyme.
- 35. Complex lV: cytochrome oxidase (cytochrome- oxygen oxidoreductases) It transfers electrons from cytochrome C to oxygen.It needs cyt a and cyt a3 as co enzyme. In this way hydrogen atoms are successively transferred through the respiratory chain to oxygen to produce water and energy.
- 37. Redox reactions in photosynthesis
- 38. It is not a single simple step process but is a complex one that is completed by a series of reactions. 1- The light dependent reaction( Light reaction). 2- The light independent reaction ( dark reaction). Light dependent phase ( oxidation phase). Photolysis Phase of photosynthesis during which light energy i s absorbed by chlorophyll and other photosynthetic pigments. It is then converted into chemical energy .Due to this energy conversion ,reducing and assimilating power i n the form of NADPH2(NADPH H+) and ATP are prod uced . Products of light reaction : ATP , NADPH.
- 41. THANK YOU