Genetic variations in gpcr
•Als PPTX, PDF herunterladen•
98 gefällt mir•10,801 views
know about diseases associated with alterations in GPCR
Melden
Teilen
Melden
Teilen
Empfohlen
Introduction to Terms, Cell Viability Assay, Glucose Uptake Assay, Calcium Uptake Assay
Presented by
B. KRANTHI KUMAR
Department of Pharmacology PRINCIPLE AND APPLICATIONS OF CELL VIABILITY, GLUCOSE UPTAKE AND CALCIUM UPT...

PRINCIPLE AND APPLICATIONS OF CELL VIABILITY, GLUCOSE UPTAKE AND CALCIUM UPT...Raghavendra institute of pharmaceutical education and research .
Empfohlen
Introduction to Terms, Cell Viability Assay, Glucose Uptake Assay, Calcium Uptake Assay
Presented by
B. KRANTHI KUMAR
Department of Pharmacology PRINCIPLE AND APPLICATIONS OF CELL VIABILITY, GLUCOSE UPTAKE AND CALCIUM UPT...

PRINCIPLE AND APPLICATIONS OF CELL VIABILITY, GLUCOSE UPTAKE AND CALCIUM UPT...Raghavendra institute of pharmaceutical education and research .
Introduction to Screening Models of Anti-Atherosclerosis
Atherosclerosis, Screening models, In vitro models, In vivo models
Presented by
SHAIK FIRDOUS BANU
Department of Pharmacology Screening Models of Anti-Atherosclerosis

Screening Models of Anti-AtherosclerosisRaghavendra institute of pharmaceutical education and research .
Screening models on behavioral and muscle coordination convertedScreening models on behavioral and muscle coordination converted

Screening models on behavioral and muscle coordination convertedB V V S Hanagal Shri Kumareshwar College of Pharmacy, Bagalkote
Weitere ähnliche Inhalte
Was ist angesagt?
Introduction to Screening Models of Anti-Atherosclerosis
Atherosclerosis, Screening models, In vitro models, In vivo models
Presented by
SHAIK FIRDOUS BANU
Department of Pharmacology Screening Models of Anti-Atherosclerosis

Screening Models of Anti-AtherosclerosisRaghavendra institute of pharmaceutical education and research .
Screening models on behavioral and muscle coordination convertedScreening models on behavioral and muscle coordination converted

Screening models on behavioral and muscle coordination convertedB V V S Hanagal Shri Kumareshwar College of Pharmacy, Bagalkote
Was ist angesagt? (20)
Neurotransmitters/General aspect and steps involved in neurotransmission.pptx

Neurotransmitters/General aspect and steps involved in neurotransmission.pptx
Assignment on Preclinical Screening of Immunomodulators

Assignment on Preclinical Screening of Immunomodulators
Screening models for aphrodisiac agents and anti fertility agents

Screening models for aphrodisiac agents and anti fertility agents
Principle and applications of glucose uptake and calcium influx assay by vivek

Principle and applications of glucose uptake and calcium influx assay by vivek
genetic variations and its role in health/ pharmacology

genetic variations and its role in health/ pharmacology
Screening methods of immunomodulators by shivam diwaker

Screening methods of immunomodulators by shivam diwaker
Screening Methods for behavioural and muscle Coordination

Screening Methods for behavioural and muscle Coordination
Screening models on behavioral and muscle coordination converted

Screening models on behavioral and muscle coordination converted
EXTRAPOLATION OF IN VITRO DATA TO PRECLINICAL TO HUMANS 

EXTRAPOLATION OF IN VITRO DATA TO PRECLINICAL TO HUMANS
Genetic variation and its role in health and pharmacology

Genetic variation and its role in health and pharmacology
Andere mochten auch
Andere mochten auch (14)
Pharmacogenetics and individual variation of drug response

Pharmacogenetics and individual variation of drug response
Sedatives and hypnotics drugs ppt by kashikant yadav

Sedatives and hypnotics drugs ppt by kashikant yadav
Ähnlich wie Genetic variations in gpcr
Ähnlich wie Genetic variations in gpcr (20)
genetic variation in gpcr and its disease due to variation

genetic variation in gpcr and its disease due to variation
Basic concepts of G – protein coupled receptor.pptx

Basic concepts of G – protein coupled receptor.pptx
Kürzlich hochgeladen
☑️░ 9630942363 ░ CALL GIRLS ░ VIP ░ ESCORT ░ SERVICES ░ AGENCY ░
9630942363 THE GENUINE ESCORT AGENCY VIP LUXURY CALL GIRLS
HIGH CLASS MODELS CALL GIRLS GENUINE ESCORT BOOK
BOOK APPOINTMENT - 9630942363 THE GENUINE ESCORT AGENCY
BEST VIP CALL GIRLS & ESCORTS SERVICE 9630942363 VIP CALL GIRLS ALL TYPE WOMEN AVAILABLE
INCALL & OUTCALL BOTH AVAILABLE BOOK NOW
9630942363 VIP GENUINE INDEPENDENT ESCORT AGENCY
VIP PRIVATE AUNTIES
BEAUTIFUL LOOKING HOT AND SEXT GIRLS AND PARTY TYPE GIRLS YOU WANT SERVICE THEN CALL THIS NUMBER 9630942363
ROOM ALSO PROVIDE HOME & HOTELS SERVICE
FULL SAFE AND SECURE WORK
WITHOUT CONDOMS, ORAL, SUCKING, LIP TO LIP, ANAL, BACK SHOTS, SEX 69, WITHOUT BLOWJOB AND MUCH MORE
FOR BOOKING
9630942363Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...

Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...GENUINE ESCORT AGENCY
Kürzlich hochgeladen (20)
Call Girls Bhubaneswar Just Call 9907093804 Top Class Call Girl Service Avail...

Call Girls Bhubaneswar Just Call 9907093804 Top Class Call Girl Service Avail...
Call Girls Aurangabad Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Aurangabad Just Call 8250077686 Top Class Call Girl Service Available
Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...

Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...
Top Rated Bangalore Call Girls Ramamurthy Nagar ⟟ 9332606886 ⟟ Call Me For G...

Top Rated Bangalore Call Girls Ramamurthy Nagar ⟟ 9332606886 ⟟ Call Me For G...
College Call Girls in Haridwar 9667172968 Short 4000 Night 10000 Best call gi...

College Call Girls in Haridwar 9667172968 Short 4000 Night 10000 Best call gi...
Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...

Pondicherry Call Girls Book Now 9630942363 Top Class Pondicherry Escort Servi...
The Most Attractive Hyderabad Call Girls Kothapet 𖠋 9332606886 𖠋 Will You Mis...

The Most Attractive Hyderabad Call Girls Kothapet 𖠋 9332606886 𖠋 Will You Mis...
Top Rated Bangalore Call Girls Mg Road ⟟ 9332606886 ⟟ Call Me For Genuine S...

Top Rated Bangalore Call Girls Mg Road ⟟ 9332606886 ⟟ Call Me For Genuine S...
VIP Service Call Girls Sindhi Colony 📳 7877925207 For 18+ VIP Call Girl At Th...

VIP Service Call Girls Sindhi Colony 📳 7877925207 For 18+ VIP Call Girl At Th...
(Low Rate RASHMI ) Rate Of Call Girls Jaipur ❣ 8445551418 ❣ Elite Models & Ce...

(Low Rate RASHMI ) Rate Of Call Girls Jaipur ❣ 8445551418 ❣ Elite Models & Ce...
Call Girls Agra Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Agra Just Call 8250077686 Top Class Call Girl Service Available
Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time

Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time
Call Girls Visakhapatnam Just Call 8250077686 Top Class Call Girl Service Ava...

Call Girls Visakhapatnam Just Call 8250077686 Top Class Call Girl Service Ava...
Call Girls Nagpur Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Nagpur Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Cuttack Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Cuttack Just Call 9907093804 Top Class Call Girl Service Available
Top Rated Bangalore Call Girls Richmond Circle ⟟ 9332606886 ⟟ Call Me For Ge...

Top Rated Bangalore Call Girls Richmond Circle ⟟ 9332606886 ⟟ Call Me For Ge...
Call Girls Coimbatore Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Coimbatore Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Faridabad Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Faridabad Just Call 9907093804 Top Class Call Girl Service Available
O898O367676 Call Girls In Ahmedabad Escort Service Available 24×7 In Ahmedabad

O898O367676 Call Girls In Ahmedabad Escort Service Available 24×7 In Ahmedabad
Night 7k to 12k Navi Mumbai Call Girl Photo 👉 BOOK NOW 9833363713 👈 ♀️ night ...

Night 7k to 12k Navi Mumbai Call Girl Photo 👉 BOOK NOW 9833363713 👈 ♀️ night ...
Genetic variations in gpcr
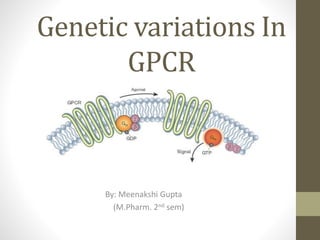
- 1. Genetic variations In GPCR By: Meenakshi Gupta (M.Pharm. 2nd sem)
- 2. What is G-protein?? • also known as guanine nucleotide-binding proteins. • family of protein that act as a molecular switches inside the cell. • Activity regulated by factors that controls their ability to bind to and hydrolyze GTP into GDP. • When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPase. • There are two classes of G proteins: a. monomeric small GTPase b. heterotrimeric G protein complexes (alpha (α), beta (β) and gamma (γ) subunit)
- 3. G-Protein Coupled Receptor 7 trans membrane helices connected by alternating cytosolic and extra cellular loop C terminal: inside the cell N terminal : extra cellular region Extra cellular portion has unique messenger binding site Cytosolic loop allow receptor to interact with G protein. The eventual effect of agonist -induced activation is a change in the relative orientations of the TM helices (likened to a twisting motion) leading to a wider intracellular surface and "revelation" of residues of the intracellular helices and TM domains crucial to signal transduction function (i.e., G-protein coupling). Inverse agonists and antagonists may also bind to a number of different sites, but the eventual effect must be prevention of this TM helix reorientation
- 5. Genetic variations may be due to 1.Sequence variations of the human genome • Introduces variability in genetic make-up • Suspected to play a main role in diseases & variable response in drug therapy • Polymorphism- refers to sequence variation leading to occurrence of two or more clearly different forms. • Single nucleotide polymorphism accounts for approx. 80% of all sequence variations.
- 6. 2. Structure & function of GPCRs • Comprises a large class of membrane proteins – encoded by approx. 600 human genes. • Molecular architecture might permit the prediction of functionally relevant domains where sequence variations are more likely to alter receptor function. • Normally, TM domains are highly conserved, the loops are variable in sequence & length, &the C- and N-terminals tails represents the most diverse elements.
- 7. 3. GPCR coupling to G proteins and other signaling pathways • GPCR thought to be couple to heterotrimeric G proteins composed of α, β and γ subunits. It display considerable heterogenecity, with a predicted number of 27 different α, 5 β and 13 γ subunits. • Main sites of contact between receptor and G proteins include the third intracellular loop, but i1, i2 and the C- terminus have also been reported to contribute G protein coupling • Proteins like protein kinases, arrestin & phosphatases modulates receptor functions at distinct domains that are possible targets for polymorphic effects.
- 8. 4. GPCR binding pockets • Ca++, acetyl choline, glutamate, bradykinin, prostaglandins, & the large polypeptide FSH bind to the same site. • Distinct binding sites appear to exist, either embedded within the pocket formed by the 7- TMD bundle within the membrane, at pockets formed by the extracellular loops, or in the N- terminus. • The thrombin receptor family represents a special case whereas the protease activity of the ligand thrombin cleaves a portion of the N- terminus. The newly generated N-terminus then serves as a tethered ligand.
- 9. • GPCRs appear to be activated by ligand binding to many different sites of the protein . At the opioid receptors, peptide endorphins bind primarily to the extracellular loops, whereas opioid alkaloids dock deep into the 7-TMD core. Human μ opioid receptor
- 10. • Sequence variation in the receptor protein can affect ligand binding or the structural integrity of the receptor , indirectly changing ligand binding . 5. Spontaneous GPCR signalling • Exchange of single amino acid residues can lead to constitutive receptor activation. • Considerable number of human polymorphisms enhance signalling (gain of function) or even activate the receptor constitutively, causing serious genetic disorders.
- 11. 6. Multiple receptor conformations with distinct functions • GPCRs are flexible structures and may accommodate ligands in various ways. It exists in multiple conformations. Discrete signalling pathways are triggered by discrete conformational states of GPCR
- 12. Sequence variations of GPCRs and associated diseases
- 13. Impaired or enhanced agonist signalling efficacy Several inactivating sequence variants of peptide receptors have been associated with congenital disorders. For example, 1. A point mutation causing truncation of thyrotropin stimulating hormone receptor leads to leydig’s cell hyperplasia.(activating mutation) Truncated TM5, D578G, T398M
- 14. 2. Inactivating mutations of the ACTH receptor are associated with familial glucocorticoid deficiency . The mutation occurs in the large N-terminus , the binding site for glycoprotein hormone receptor, leading to toxic multinodular goiter. S120R, R201Stop, S74I, V254C
- 15. V2 vasopressin recptors • A number of mutations in the gene encoding the V2 vasopressin receptor leads to functionally inactive receptor protein and are causative for nephrogenic diabetes insipidus.(missense mutations) • This a clear indication that receptor activity depends on intact signalling pathways. (multiple SNPs; decreased ligand binding; R113W; R137H)
- 17. Thromboxane A2 Receptor • This receptor performs an essential role in haemostasis by inducing platelet aggregation. An R60L amino acid substitution in the first cytoplasmic loop of TBXA2 receptor causes a dominantly inherited bleeding disorder characterised by defective platelet response to TBXA2. This leads to decreased agonist-induced second messenger formation.
- 19. P2Y 12ADP Receptor • This receptor sub-type is shown to be the target for anti-thrombotic drugs such as ticlodipine & clopidogrel. 2-nucleotide deletion in a region mapping to the end of TMD6, associated with a rare bleeding disorder.
- 20. Chemokine reeptors • Fusin and CKR5 have been identified as a co- receptors for the cellular entry of HIV. Similarly , certain chemokines were found to block HIV entry into cells. • Natural resistance can be either by high endogenous levels of chemokines or by mutations of the receptors. • A 32 bp deletion in CKR5 leading to a frame shift and a non functional protein appeared to protect homozygous carriers against HIV infection & blocking its entry.
- 21. • Val 64 substitution with Ile was shown to result in heterodimerisation of CCR2 with CCR5 or CXCR4, thereby promoting resistance to AIDS.
- 22. Biogenic amine receptors • The R16G substitution in the β2 adrenoreceptors has been associated with nocturnal asthma whereas W64R in the β3 receptor expressed in adipocytes are involved in energy metabolism – is linked with obesity.
- 23. Sequence variants of human G coupled receptors
- 24. receptors Variant/Allele Disease/ Phenotype cellular mechanism/ Event Β1 adrenergic receptor R16G Nocturnal asthma; Enhanced agonist promoted down regulation of receptor Β3 adrenergic receptors W64R Obesity Luteinising hormone Truncated TM5 D578G Leydig’s cell hyperplasia; Precocious puberty in male children FSH A189V Ovarian dysgenesis; Altered protein folding; inactivation of receptor Thyrotropin (TSH) S120R, R201Stop, S74I, V254C Glucocorticoid deficiency; altered/ loss of receptor function/ reduced expression ACTH D727E Altered receptor function/conformation; Toxic multinodular goiter Vasopressin V2 receptor Multiple SNPs Nephrogenic diabetes insipidus;
- 25. Decreased ligand binding; reduced expression of receptor Chemokine receptors CCR2 CCR3 CCR5 V64I R275Q,L351P CCR5P1 alleles Delayed progression of AIDS Unknown functional change or influence on disease Increased progression of AIDS Thromboxane A2 R60L Bleeding disorder ADP receptor P2Y12 Del of 2 nt (TTCATT) in coding region (end of TMD6) Bleeding disorder
- 26. THANK YOU
