Acid and Base Definitions.ppt
•Als PPT, PDF herunterladen•
0 gefällt mir•14 views
This subject is known as chemistry which is a sub branch of science. The ppt is about acids and bases and there types, definitions and etc
Melden
Teilen
Melden
Teilen
Empfohlen
Weitere ähnliche Inhalte
Ähnlich wie Acid and Base Definitions.ppt
Ähnlich wie Acid and Base Definitions.ppt (20)
Acid base reactions BY MUHAMMAD FAHAD ANSARI 12IEEM 14

Acid base reactions BY MUHAMMAD FAHAD ANSARI 12IEEM 14
Chemistry - Chp 19 - Acids, Bases, and Salt - PowerPoints

Chemistry - Chp 19 - Acids, Bases, and Salt - PowerPoints
B sc_I_General chemistry U-II Ionic equilibria in aqueous solution 

B sc_I_General chemistry U-II Ionic equilibria in aqueous solution
B sc i chemistry i u ii ionic equilibria in aqueous solution a

B sc i chemistry i u ii ionic equilibria in aqueous solution a
Kürzlich hochgeladen
Kürzlich hochgeladen (20)
Selaginella: features, morphology ,anatomy and reproduction.

Selaginella: features, morphology ,anatomy and reproduction.
pumpkin fruit fly, water melon fruit fly, cucumber fruit fly

pumpkin fruit fly, water melon fruit fly, cucumber fruit fly
FAIRSpectra - Enabling the FAIRification of Spectroscopy and Spectrometry

FAIRSpectra - Enabling the FAIRification of Spectroscopy and Spectrometry
Call Girls Ahmedabad +917728919243 call me Independent Escort Service

Call Girls Ahmedabad +917728919243 call me Independent Escort Service
POGONATUM : morphology, anatomy, reproduction etc.

POGONATUM : morphology, anatomy, reproduction etc.
FAIRSpectra - Enabling the FAIRification of Analytical Science

FAIRSpectra - Enabling the FAIRification of Analytical Science
The Mariana Trench remarkable geological features on Earth.pptx

The Mariana Trench remarkable geological features on Earth.pptx
Porella : features, morphology, anatomy, reproduction etc.

Porella : features, morphology, anatomy, reproduction etc.
Acid and Base Definitions.ppt
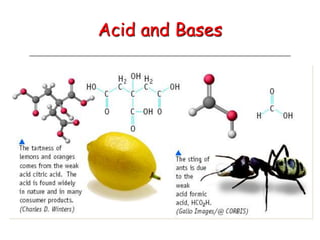
- 4. Acids Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain metals to produce hydrogen gas. React with carbonates and bicarbonates to produce carbon dioxide gas Have a bitter taste. Feel slippery. Many soaps contain bases. Bases
- 5. Some Properties of Acids Produce H+ (as H3O+) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) Taste sour Corrode metals Electrolytes React with bases to form a salt and water pH is less than 7 Turns blue litmus paper to red “Blue to Red ACID”
- 6. Anion Ending Acid Name -ide hydro-(stem)-ic acid -ate (stem)-ic acid -ite (stem)-ous acid Acid Nomenclature Review No Oxygen w/Oxygen An easy way to remember which goes with which… “In the cafeteria, you ATE something Icky”
- 7. Acid Nomenclature Flowchart hydro- prefix -ic ending 2 elements -ate ending becomes -ic ending -ite ending becomes -ous ending no hydro- prefix 3 elements ACIDS start with 'H'
- 8. • HBr (aq) • H2CO3 • H2SO3 hydrobromic acid carbonic acid sulfurous acid Acid Nomenclature Review
- 9. Some Properties of Bases Produce OH- ions in water Taste bitter, chalky Are electrolytes Feel soapy, slippery React with acids to form salts and water pH greater than 7 Turns red litmus paper to blue “Basic Blue”
- 10. Some Common Bases NaOH sodium hydroxide lye KOH potassium hydroxide liquid soap Ba(OH)2 barium hydroxide stabilizer for plastics Mg(OH)2 magnesium hydroxide “MOM” Milk of magnesia Al(OH)3 aluminum hydroxide Maalox (antacid)
- 11. Acid/Base definitions • Definition 1: Arrhenius (traditional) Acids – produce H+ ions (or hydronium ions H3O+) in water Bases – produce OH- ions in water (problem: some bases don’t have hydroxide ions!)
- 12. Arrhenius acid is a substance that produces H+ (H3O+) in water Arrhenius base is a substance that produces OH- in water
- 13. Acid/Base Definitions • Definition 2: Brønsted Lowry Acids : proton donor Bases : proton acceptor A “proton” is really just a hydrogen atom that has lost it’s electron!
- 14. A Brønsted-Lowry acid is a proton donor A Brønsted-Lowry base is a proton acceptor acid conjugate base base conjugate acid
- 15. ACID-BASE THEORIES The Brønsted definition means NH3 is a BASE in water and water is itself an ACID Base Acid Acid Base NH4 + + OH- NH3 + H2O
- 16. Amphoteric Substances • A substance that is Amphoteric can act as either an acid or a base. • In the previous slide, water acted as an acid. • In the following example, water acts as a base. HCl (g) + H2O (l) H3O+ (aq) + Cl- (aq) acid base conj. acid conj. base
- 17. Conjugate Pairs
- 18. Acid-Base Behavior • Consider a compound having the formula HOX. • If X is highly electronegative, it will have a strong attraction for the electrons shared with O. o The O, will in turn, pull strongly on the electrons held shared with H. o This H will then be easily lost = acid • If X has a low electro negativity, the oxygen will pull the electrons away from X. o The hydrogen will remain joined to the oxygen. o Since the O and H can easily remain together, it is likely that OH- will be formed = base • Nonmetals tend to have high EN = acids • Metals tend to have low EN = bases
- 19. Acids & Base Definitions Lewis acid: A substance that accepts an electron pair Lewis base: A substance that donates an electron pair Definition 3: Lewis
- 20. Formation of hydronium ion is also an excellent example. Lewis Acids & Bases •Electron pair of the new O-H bond originates on the Lewis base. H H H BASE •• • ••• O—H O—H H+ ACID
