Computational and Experimental Studies of MTO Catalyzed Olefin Hydrogenation
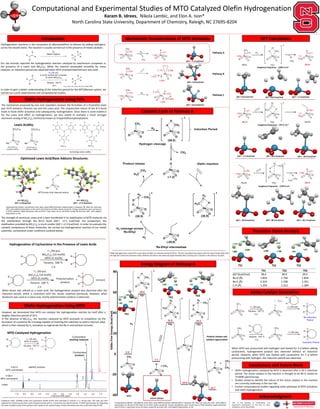
- 1. The strength of aluminum Lewis acid is best manifested in its stabilization of MTO molecule via the coordination through the Re=O bond (∆Go= -17.5 kcal/mol). For comparison, the stabilization provided by B(C6F5)3 is much smaller (∆Go= +2.3 kcal/mol). In order to evaluate the catalytic competency of these molecules, we carried out hydrogenation reaction of our model substrate, cyclooctene under conditions outlined below: Computational and Experimental Studies of MTO Catalyzed Olefin Hydrogenation Karam B. Idrees, Nikola Lambic, and Elon A. Ison* North Carolina State University, Department of Chemistry, Raleigh, NC 27695-8204 Hydrogenation reactions is the conversion of alkenes(olefins) to alkanes by adding hydrogens across the double bond. The reaction is usually carried out in the presence of metal catalysts: However, we discovered that MTO can catalyze the hydrogenation reaction by itself after a lengthy induction period of 20 h. In the absence of B(C6F5)3, the reaction catalyzed by MTO proceeds to completion via the formation of a putative Re-H analog capable of inserting the substrate to yield a rhenium alkyl, which is then cleaved by H2 activation to regenerate the Re-H and achieve turnover. TS1 TS2 Introduction Olefin Hydrogenation Using FLPs Mechanistic Considerations of MTO Activation Catalytic Cycle of Pathway A Energy Diagram of Pathway A Transition States Analysis Conclusions and Future Work -REU at the Interface of Computations and Experiments coordinators: Dr. Elon Ison, Dr. Elena Jakubikova, and Dr. Reza Ghiladi. DFT Calculations B(C6F5)3 Al(C6F5)3 Increasing Lewis acidity LA= Al(C6F5)3 ∆Go= -17.5 kcal/mol Optimized Lewis Acid/Base Adducts Structures: MTO/ B(C6F5)3 Acid/Base adduct MTO/ Al(C6F5)3 Acid/Base adduct Lewis Acidity: -40 -20 0 20 40 60 80 GibbsFreeEnergy(kcal/mol) ReactionCoordinate ∆Go= 0.0 kcal/mol ∆G‡= 58.6 kcal/mol ∆Go= -7.2 kcal/mol Imaginary frequency: -1284.4 cm-1 Imaginary frequency: -547.6 cm-1 ∆Go= -7.2 kcal/mol ∆G‡= 30.9 kcal/mol ∆Go= -29.6 kcal/mol ∆Go= -29.6 kcal/mol ∆G‡= 30.5 kcal/mol ∆Go= -34.7 kcal/mol Imaginary frequency: -1259.6 cm-1 Olefin Hydrogenation Using MTO TS1 TS2 TS3 ∆G‡ (kcal/mol) 58.6 30.9 30.5 Re-H (Å) 1.853 1.756 1.854 Re-C (Å) 2.323 2.339 2.351 C-H (Å) 1.354 1.512 1.364 Conditions: MTO (0.0046 mmol) and cyclooctene (0.092 mmol) were dissolved In toluene in a J-Young tube. The tube was then subjected to 3 freeze pump thaw cycles and pressurized with H2. Conversion was determined by 1H NMR spectroscopy by integrating the ratios of olefinic peak of the product with respect to the reactant peak. Product formation was also confirmed using GC-MS. Olefin hydrogenation using MTO is only observed after an induction period of 20 hrs. The first calculated step agrees with the experimental data with the high ∆G≠ of the first transition state. Methane formation was observed experimentally when carrying out a reaction in the absence of olefin. • Olefin hydrogenation catalyzed by MTO is observed after a 20 h induction period. The active catalyst in this reaction is thought to be Re-H, based on 1H NMR spectroscopy. • Studies aimed to identify the nature of the active catalyst in the reaction are currently underway in the Ison lab. • Further computational studies regarding other pathways of MTO activation and olefin hydrogenation. Acknowledgment Computational details: Calculations were done using M06 functional implemented in Gaussian 09. Basis set used was SDD with added f polarization on Re and 6-31G (d,p) on all other atoms except Re. Energy calculations were carried out in PCM solvation model (benzene) with 6-311G++ (d,p) basis set on all atoms except Re and was SDD with added f polarization on Re. ∆Go= 4.8 kcal/mol ∆Go= 13.0 kcal/mol ∆Go= -7.2 kcal/mol Pathway A Pathway B Pathway C Computational details: Calculations were done using M06 functional implemented in Gaussian 09. Basis set used was SDD with added f polarization on Re and 6-31G (d,p) on all other atoms except Re. Energy calculations were carried out in PCM solvation model (benzene) with 6-311G++ (d,p) basis set on all atoms except Re and was SDD with added f polarization on Re. Active Catalyst Generation TS1 TS2 TS3 Our lab recently reported the hydrogenation reaction catalyzed by oxorhenium complexes in the presence of a Lewis acid B(C6F5)3. While the reaction proceeded smoothly for many catalysts, an induction period was observed when MTO (methyltrioxorhenium) was used . J. Am. Chem. Soc., 2016, 138 (14), pp 4832–4842 In order to gain a better understanding of the induction period for the MTO/Borane system, we carried out a joint experimental and computational studies. The mechanism proposed by Ison and coworkers involves the formation of a frustrated Lewis pair (FLP) between rhenium oxo and the Lewis acid. The unquenched nature of the B-O bond leads to facile olefin activation and subsequently, hydrogenation. Since there is some evidence for the Lewis acid effect on hydrogenation, we also aimed to evaluate a much stronger aluminum analog of B(C6F5)3 commonly known as tris(pentafluorophenyl)alane. LA= B(C6F5)3 ∆Go= 2.3kcal/mol Hydrogenation of Cyclooctene in the Presence of Lewis Acids When boron was utilized as a Lewis acid, the hydrogenation product was observed after the induction period, which is consistent with the results reported previously. However, when aluminum was used as a Lewis acid, mostly polymerization product is observed. MTO Catalyzed Hydrogenation MTO Active catalyst Product release and catalyst regeneration TS3 When MTO was pressurized with hydrogen and heated for 3 d before adding cyclooctene, hydrogenated product was observed without an induction period. However, when MTO was heated with cyclooctene for 3 d before pressurizing with hydrogen, the induction period was observed.