Adc drugs some challenges to be solved
•
0 gefällt mir•75 views
Despite the increasing number of ADC approvals, there are still challenges for ADCs that have demonstrated superior safety and efficacy in the clinic. What're the challenges?
Melden
Teilen
Melden
Teilen
Downloaden Sie, um offline zu lesen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (19)
Art 923 rev-c-updating investor presentation on valeritas website_final_12.05.16

Art 923 rev-c-updating investor presentation on valeritas website_final_12.05.16
The immune checkpoint landscape in 2015: combination therapy

The immune checkpoint landscape in 2015: combination therapy
The basic knowledge of Antibody-drug conjugates (ADC) - Creative Biolabs

The basic knowledge of Antibody-drug conjugates (ADC) - Creative Biolabs
Epigenetics Diagnostic Market Size, Share, Growth, Trends and Forecast Report...

Epigenetics Diagnostic Market Size, Share, Growth, Trends and Forecast Report...
Global cancer tyrosine kinase inhibitors market & clinical pipeline outlo...

Global cancer tyrosine kinase inhibitors market & clinical pipeline outlo...
Antibody drug conjugates current status and future perspectives

Antibody drug conjugates current status and future perspectives
Global cancer kinase inhibitors market & pipeline insight

Global cancer kinase inhibitors market & pipeline insight
Personalized medicine - Mathura Shanmugasundaram PhD

Personalized medicine - Mathura Shanmugasundaram PhD
First immunotherapy for early stage triple-negative breast cancer

First immunotherapy for early stage triple-negative breast cancer
Cancer immunomodulators market & pipeline insight 2020

Cancer immunomodulators market & pipeline insight 2020
Mechanisms and applications of apoptosis based and molecular

Mechanisms and applications of apoptosis based and molecular
Ähnlich wie Adc drugs some challenges to be solved
Abstract
Gene therapy as a modern therapeutic approach has not yet advanced to a globally-approved therapeutic approach. Lack of adequate reliable gene delivery system seems to be one of the major reasons from the pharmaceutical biotechnology point of view. Main obstacles delaying successful application of human gene therapy are presented in this review. The unique advantages of non-biological gene carriers as compared to their biological counterparts make them ideal alternatives for overcoming extra- and intracellular barriers in a more safely manner. We, therefore, highlight the significant contributions in non-biological gene delivery and favorable characteristics of different design attitudes with focus on in vivo approaches. Bypassing the rapid extracellular enzymatic degradation of genetic materials is covered in extracellular segment of this review with emphasis on PEGylated and targeted formulations. The successful approaches to pave the rest of the way from cellular uptake to intracellular transfer and gene expression of unpacked DNA are also discussed. From these approaches, we emphasize more on optimization of cationic-based polymers and dendrimers, developing newly designed membrane-effective components, and adjusting the hydrophilic-hydrophobic balance of the synthesized vectorsNon-biological gene carriers designed for overcoming the major extra- and int...

Non-biological gene carriers designed for overcoming the major extra- and int...Nanomedicine Journal (NMJ)
Ähnlich wie Adc drugs some challenges to be solved (20)
Antibody drug conjugates for cancer therapy - prospects and challenges htp

Antibody drug conjugates for cancer therapy - prospects and challenges htp
Aptamer-Drug Conjugate (ApDC) Current Research Progress.pdf

Aptamer-Drug Conjugate (ApDC) Current Research Progress.pdf
Monoclonal Antibodies Limitations & Potential Improvement Strategies.pdf

Monoclonal Antibodies Limitations & Potential Improvement Strategies.pdf
Bioanalytical Method Development and Validation of Biosimilars: Lessons Learned

Bioanalytical Method Development and Validation of Biosimilars: Lessons Learned
9 Types of Drug Conjugates Overview ADC、RDC、ISAC、SMDC、AOC.pdf

9 Types of Drug Conjugates Overview ADC、RDC、ISAC、SMDC、AOC.pdf
Summary of Targeted Protein Degradation in Clinical Trials.pdf

Summary of Targeted Protein Degradation in Clinical Trials.pdf
Global antibody-drug-conjugate-adc-clinical-trial-review

Global antibody-drug-conjugate-adc-clinical-trial-review
MedImmune Industrial Placement Student Programme 2016

MedImmune Industrial Placement Student Programme 2016
Non-biological gene carriers designed for overcoming the major extra- and int...

Non-biological gene carriers designed for overcoming the major extra- and int...
PROTAC Technology: An Effective Targeted Protein Degrader.pdf

PROTAC Technology: An Effective Targeted Protein Degrader.pdf
Accelerate innovation and manufacturing in cell and gene therapy.pptx

Accelerate innovation and manufacturing in cell and gene therapy.pptx
Summary of PROTAC Degraders in Clinical Trials.pdf

Summary of PROTAC Degraders in Clinical Trials.pdf
Mehr von DoriaFang
Mehr von DoriaFang (20)
Cyclic Peptides Current Status & Future Prospects.pdf

Cyclic Peptides Current Status & Future Prospects.pdf
Antibody–Oligonucleotide Conjugates (AOCs) in Clinical Trials.pdf

Antibody–Oligonucleotide Conjugates (AOCs) in Clinical Trials.pdf
Alzheimer's Disease Drug Development Aducanumab, Lecanemab & Donanemab.pdf

Alzheimer's Disease Drug Development Aducanumab, Lecanemab & Donanemab.pdf
Claudin6 (CLDN6) A Emerging Target For Solid Tumor.pdf

Claudin6 (CLDN6) A Emerging Target For Solid Tumor.pdf
ROR1 ADCs in Clinical Trials MK-2140, NBE-002 & CS5001.pdf

ROR1 ADCs in Clinical Trials MK-2140, NBE-002 & CS5001.pdf
Trophoblast Glycoprotein (TPGB5T4) A New Target For ADC Drugs.pdf

Trophoblast Glycoprotein (TPGB5T4) A New Target For ADC Drugs.pdf
DS-8201 (Enhertu) A Potential ADC Drug Targeting HER2.pdf

DS-8201 (Enhertu) A Potential ADC Drug Targeting HER2.pdf
List of New Anti-cancer Drugs Approved By FDA In The First Half of 2023.pdf

List of New Anti-cancer Drugs Approved By FDA In The First Half of 2023.pdf
Overview of Oral Delivery Strategies for Peptides.pdf

Overview of Oral Delivery Strategies for Peptides.pdf
Summary of ADC Targets For Solid Tumors & Hematological Tumors.pdf

Summary of ADC Targets For Solid Tumors & Hematological Tumors.pdf
New Oncology Trends ADCs, Bispecific Antibodies & CAR-T Cell.pdf

New Oncology Trends ADCs, Bispecific Antibodies & CAR-T Cell.pdf
Bispecific Antibody-drug Conjugate Drugs In Clinical or Preclinical.pdf

Bispecific Antibody-drug Conjugate Drugs In Clinical or Preclinical.pdf
Nectin-4 New Antibody-Drug Conjugate (ADC) Target.pdf

Nectin-4 New Antibody-Drug Conjugate (ADC) Target.pdf
Kürzlich hochgeladen
Falcon stands out as a top-tier P2P Invoice Discounting platform in India, bridging esteemed blue-chip companies and eager investors. Our goal is to transform the investment landscape in India by establishing a comprehensive destination for borrowers and investors with diverse profiles and needs, all while minimizing risk. What sets Falcon apart is the elimination of intermediaries such as commercial banks and depository institutions, allowing investors to enjoy higher yields.Falcon Invoice Discounting: The best investment platform in india for investors

Falcon Invoice Discounting: The best investment platform in india for investorsFalcon Invoice Discounting
Kürzlich hochgeladen (20)
UAE Bur Dubai Call Girls ☏ 0564401582 Call Girl in Bur Dubai

UAE Bur Dubai Call Girls ☏ 0564401582 Call Girl in Bur Dubai
Escorts in Nungambakkam Phone 8250092165 Enjoy 24/7 Escort Service Enjoy Your...

Escorts in Nungambakkam Phone 8250092165 Enjoy 24/7 Escort Service Enjoy Your...
Berhampur CALL GIRL❤7091819311❤CALL GIRLS IN ESCORT SERVICE WE ARE PROVIDING

Berhampur CALL GIRL❤7091819311❤CALL GIRLS IN ESCORT SERVICE WE ARE PROVIDING
PARK STREET 💋 Call Girl 9827461493 Call Girls in Escort service book now

PARK STREET 💋 Call Girl 9827461493 Call Girls in Escort service book now
Berhampur Call Girl Just Call 8084732287 Top Class Call Girl Service Available

Berhampur Call Girl Just Call 8084732287 Top Class Call Girl Service Available
Chennai Call Gril 80022//12248 Only For Sex And High Profile Best Gril Sex Av...

Chennai Call Gril 80022//12248 Only For Sex And High Profile Best Gril Sex Av...
Falcon Invoice Discounting: The best investment platform in india for investors

Falcon Invoice Discounting: The best investment platform in india for investors
Lucknow Housewife Escorts by Sexy Bhabhi Service 8250092165

Lucknow Housewife Escorts by Sexy Bhabhi Service 8250092165
JAJPUR CALL GIRL ❤ 82729*64427❤ CALL GIRLS IN JAJPUR ESCORTS

JAJPUR CALL GIRL ❤ 82729*64427❤ CALL GIRLS IN JAJPUR ESCORTS
SEO Case Study: How I Increased SEO Traffic & Ranking by 50-60% in 6 Months

SEO Case Study: How I Increased SEO Traffic & Ranking by 50-60% in 6 Months
Falcon Invoice Discounting: Empowering Your Business Growth

Falcon Invoice Discounting: Empowering Your Business Growth
New 2024 Cannabis Edibles Investor Pitch Deck Template

New 2024 Cannabis Edibles Investor Pitch Deck Template
joint cost.pptx COST ACCOUNTING Sixteenth Edition ...

joint cost.pptx COST ACCOUNTING Sixteenth Edition ...
Durg CALL GIRL ❤ 82729*64427❤ CALL GIRLS IN durg ESCORTS

Durg CALL GIRL ❤ 82729*64427❤ CALL GIRLS IN durg ESCORTS
Adc drugs some challenges to be solved
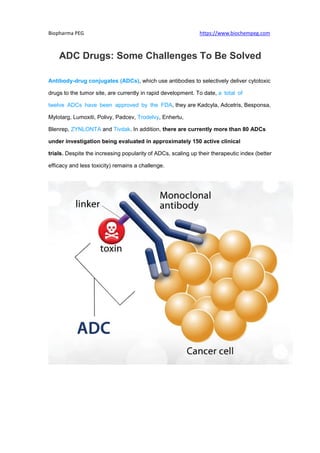
- 1. Biopharma PEG https://www.biochempeg.com ADC Drugs: Some Challenges To Be Solved Antibody-drug conjugates (ADCs), which use antibodies to selectively deliver cytotoxic drugs to the tumor site, are currently in rapid development. To date, a total of twelve ADCs have been approved by the FDA, they are Kadcyla, Adcetris, Besponsa, Mylotarg, Lumoxiti, Polivy, Padcev, Trodelvy, Enhertu, Blenrep, ZYNLONTA and Tivdak. In addition, there are currently more than 80 ADCs under investigation being evaluated in approximately 150 active clinical trials. Despite the increasing popularity of ADCs, scaling up their therapeutic index (better efficacy and less toxicity) remains a challenge.
- 2. Biopharma PEG https://www.biochempeg.com ADC can't prove itself stronger Despite the increasing number of ADC approvals, there are still challenges for ADCs that have demonstrated superior safety and efficacy in the clinic. An unexpected challenge faced by many developers during clinical evaluations is the inability of ADCs to demonstrate benefit compared to controls, such as MM-302. MM-302 is an anti-HER2 monoclonal antibody conjugated to doxorubicin. The Phase II HERMIONE trial (NCT02213744) was discontinued due to a lack of benefit from a placebo treatment. Another ADC that reported a similar situation was Rovalpitumab tesirine (RovA-T), encouraging results from the Phase I trial reported 18% ORR in assessable patients, 38% ORR in patients with high DLL3 expression (NCT01901653). However, safety and efficacy issues were raised due to the results of the Phase II trial TRINITY (NCT02674568), which did not meet the primary endpoint and reported high toxicity rates. The most common event in patients was pleural effusion, which was thought to be toxic in association with PBDE dimers. Ultimately, results from the Phase III trials TAHOE (NCT03061812) and MERU (NCT03033511) resulted in AbbVie completely discontinuing the development of RovA-T, with a lack of survival benefit compared to the control group.
- 3. Biopharma PEG https://www.biochempeg.com The design of ADC drug Off-target toxicity of ADCs One of the major challenges of ADCs is off-target toxicity, which is caused by the premature release of cytotoxic small molecules into the bloodstream. Antibodies are an awkward vehicle for transport, difficult to navigate through the tumor's alleys, and it is estimated that only 0.1% of the drug reaches the tumor tissue. In order to ensure that the
- 4. Biopharma PEG https://www.biochempeg.com other 99% of highly toxic warheads do not bring systemic toxicity, the chemical link between the warhead and the antibody must be sufficiently stable. However, in order to release the warhead in the cell without being too stable, this obviously brings trouble to drug design. The associated increased risk depends on the toxicity profile associated with cytotoxic small molecules. Off-target toxicities of maytansine (DM1) were hepatotoxicity and thrombocytopenia. MMAE was associated with the likelihood of peripheral neuropathy, neutropenia, and anemia. MMAF was associated with ocular toxicity. In terms of the metabolic characteristics of ADC, the hydrophobicity of ADC increases the clearance rate due to the high drug load of hydrophobic small molecules. An in vivo study involving a xenograft model compared the effect of a high dose of MMAE with different DARS (2, 4, and 8) conjugation against CD30 mab on ADC clearance. The results showed that the higher the drug loading, the higher the clearance rate. In this study, ADCs with a DAR of 8 were cleared fastest. Aggregation of ADCs In addition, ADCs are easy to aggregate. ADC aggregation leads to modifications that reduce its ability to bind antigens. Protein aggregation is a major obstacle to ADC development. It can occur at every stage as well as during transportation and long-term storage. The aggregation is immunogenic. In addition, protein aggregation can lead to product loss. Overall, any chemical or physical degradation can lead to structural changes in ADCs and lead to excessive protein aggregation. There are various other factors that can cause aggregation, such as frequent freezing/thawing, high protein and salt concentrations, elevated temperature, or low pH. In addition, most payloads are hydrophobic, and binding the payload at a high DAR on the protein surface can lead to excessive protein aggregation, hindering the successful development of ADCs. In some ADCs, the payload
- 5. Biopharma PEG https://www.biochempeg.com binds to Cys residues after breaking existing disulfide bonds, and hydrophobic-hydrophobic interactions increase. Drug resistance of ADCs Another challenge factor is drug resistance. The internalization of the payload and its efficacy are mainly affected by acquired resistance mechanisms, such as down-regulation of tumor cell antigen expression. Drug resistance occurs when a treatment fails or becomes less effective, or when tumor cells escape. Drug resistance can arise at the start of treatment or after drug treatment. There are many mechanisms by which drug resistance arises, some of which include: down-regulation of antigen level expression, drug efflux pumps, endocytosis and migration, defective lysosomal function, altered signaling pathways, and dysregulated apoptosis. Immunogenicity of ADCs With the increase in the types and wide application of macromolecular protein drugs, the related immunogenicity problems have gradually surfaced. Protein drugs have potential factors to induce immunogenicity, the consequences of which may affect efficacy and even be life-threatening. Antibody-drug conjugates (ADCs) have the same immunogenicity as antibody drugs, and their immunogenicity risks affect the safety and effectiveness of the drugs for patients, and may even bring fatal new diseases to patients due to ADA and endogenous protein cross. (See: Immunogenicity of ADC) Conclusion
- 6. Biopharma PEG https://www.biochempeg.com Along with the success and excitement of the ADC development process, there also have its complexities and limitations. This is an era of innovative drugs, with newer iterations of different technologies, and ADCs need more extensive clinical coverage and confirmation as more questions are studied in depth. In the future, ADC drugs will have a huge potential in the anti-tumor market. Biopharma PEG is a worldwide leader of PEG linker supplier that offers a wide array of different ADC linkers to empower our customer's advanced research. References: [1] Antibody–Drug Conjugates: The Last Decade,Pharmaceuticals 2020, 13, 245,1-31 [2] Targeting cancer with antibody-drug conjugates: Promises and challenges. MABS,2021, VOL. 13, NO. 1, [3] The Chemistry Behind ADC. Pharmaceuticals (Basel). 2021 May; 14(5): 442. [4] Immunogenicity of antibody-drug conjugates: observations across 8 molecules in 11 clinical trials. Bioanalysis. 2019 Sep;11(17):1555-1568. [5] Mechanisms of Resistance to Antibody–Drug Conjugates. Mol Cancer Ther; 15(12) December 2016