IB Chemistry on Electrolysis and Faraday's Law
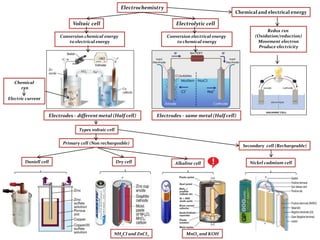
- 1. Types voltaic cell Conversion electrical energy to chemical energy Electrochemistry Electrolytic cellVoltaic cell NH4CI and ZnCI2 Chemical and electrical energy Redox rxn (Oxidation/reduction) Movement electron Produce electricity Conversion chemical energy to electrical energy Electrodes– differentmetal (Half cell) Electrodes– same metal (Half cell) Chemical rxn Electric current Daniell cell Alkaline cellDry cell Nickel cadmium cell Primary cell (Non rechargeable) MnO2 and KOH Secondary cell (Rechargeable)
- 2. Conversion electrical to chemical energy Electrochemistry ElectrolyticcellVoltaic cell Conversion chemical to electrical energy Cathode(+ve) - Reduction Cathode(-ve) - Reduction Vs Electron flow from anode (-ve) to cathode (+ve) electrode Electron flow from anode (+ve) to cathode (-ve) electrode Anode (-ve) Spontaneousrxn Non Spontaneousrxn Anode (-ve) – Oxidation Anode (+ve) – Oxidation ++ О О О О - - Zn → Zn 2+ + 2e (oxidized) Cu2+ + 2e → Cu (reduced) Zn2+ Zn2+ Zn2+ Zn2+- - - - → + + + Cu2+ Cu2+ Cu2+ -e -e + + + - - - X-→ X + -e (oxidized) X - X - X - Anode (+ve) Cathode (-ve) Cathode (+ve) -e -e Y+ + e- → Y (reduced) Y+ Y+ Y+ -e -e -e -e Anode Cathode Voltaic Cell Electrolytic Cell Anode Oxidation Negative (-ve) Oxidation Positive (+ve) Cathode Reduction Positive (+ve) Reduction Negative (-ve) Cation (+ve ion) to cathode (-ve)Anion (-ve ion) to anode (+ve)
- 3. Zn → Zn 2+ + 2e Conversion electrical to chemical energy Electrochemistry Conversion chemical to electrical energy Cathode (-ve) Reduction Vs Electron flow from anode (-ve) to cathode (+ve) electrode Electron flow from anode (+ve) to cathode (-ve) electrode Anode (-ve) Spontaneousrxn Non Spontaneousrxn Anode (+ve) Oxidation + О О - Zn → Zn 2+ + 2e (oxidized) Cu2+ + 2e → Cu (reduced) Zn2+ Zn2+ Zn2+ Zn2+ - -- -→ + + + Cu2+ Cu2+ Cu2+ -e -e + + + - - - 2Br-→ Br2 + 2e- (oxidized) Br - Br - Br - Anode (+ve) Cathode (-ve)Cathode (+ve) -e -e Pb2+ + 2e- → Pb (reduced) Pb2+ -e -e -e Cation (+ve ion) to cathode (-ve)Anion (-ve ion) to anode (+ve) 1.10Volt -e -e - - - - + + + + Anode Cathode Zn half cell (-ve) Oxidation Cu half cell (+ve) Reduction Cu2+ + 2e → Cu Zn + Cu2+ → Zn2+ + Cu 2Br- → Br2 + 2e Zn/Cu Voltaic Cell PbBr2 molten ElectrolyticCell Pb2+ + 2e → Pb PbBr2 → Pb+ Br2 Br - Br - Br - Pb2+ Pb2+ Pb2+ Pb2+ Pb2+
- 4. Conversion electrical to chemical energy Electrochemistry Conversion chemical to electrical energy Cathode (-ve) Reduction Vs Spontaneousrxn Non Spontaneousrxn Anode (+ve) Oxidation + О О - -e 1.10 Volt -e -e - - - - + + + + Anode Cathode Zn/Cu Voltaic Cell PbBr2 molten ElectrolyticCell PbBr2 → Pb+ Br2 Eθ = ??? Br - Br - Br - Pb2+ Pb2+ Pb2+ Find Eθ cell (use reduction potential) Zn 2+ + 2e ↔ Zn Eθ = -0.76V Cu2+ + 2e ↔ Cu Eθ = +0.34V Cu half cell (+ve) Reduction Zn half cell (-ve) Oxidation Zn + Cu2+ → Zn2+ + Cu Eθ = ????? Zn ↔ Zn2+ + 2e Eθ = +0.76 Cu2+ + 2e ↔ Cu Eθ = +0.34 Zn + Cu2+ → Zn 2+ + Cu Eθ = +1.10V Eθ = +1.10V +ve (spontaneous) Pb2+ + 2e ↔ Pb Eθ = -0.13V Br- + e ↔ Br - Eθ = +1.07V Find Eθ cell (use reduction potential) 2Br - ↔ Br2+ 2e Eθ = -1.07 Pb2+ + 2e ↔ Pb Eθ = -0.13 Pb2+ + 2Br - → Pb+Br2 Eθ = -1.20V Compound broken down (LYSIS) energy needed Eθ = -1.20V -ve (NON spontaneous) Conversion chemical to electrical energy Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!!
- 5. Discharge of ions 1 Cation + 1 Anion Electrolysis (Molten Salt) Oxidation ← Anode (+ve) ← Anion PbBr2 moltenElectrolytic Cell Eθ =-ve → supply +1.20v to breakdown PbBr2 → Pb+ Br2 Find Eθ cell (use reduction potential) Pb2+ + 2e ↔ Pb Eθ = -0.13 2Br - ↔ Br2+ 2e Eθ = -1.07 Pb2+ + 2Br - → Pb +Br2 Eθ = -1.20V Eθ = -1.20V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Liquid – Pb2+ and Br- ions + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn -0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ + 0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged Br- ion Br2 gas (brown gas seen) Discharged Pb2+ ion to Pb (grey deposit) 2Br - ↔ Br2+ 2e Pb2+ + 2e ↔ Pb Compound broken down (LYSIS) energy needed О О Pb2+ Br - Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Inert electrode Carbon/graphite Br - Br - Br - Pb2+ Pb2+ Pb2+
- 6. Discharge of ions 1 Cation + 1 Anion Oxidation ← Anode (+ve) ← Anion CaCI2 molten Electrolytic Cell Find Eθ cell (use reduction potential) Ca2+ + 2e ↔ Ca Eθ = -2.87 2CI - ↔ CI2+ 2e Eθ = -1.36 Ca2+ + 2CI - → Ca +CI2 Eθ = -4.23V Eθ = -4.23V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Liquid – Ca2+ and CI- ions + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 H2O + e- ↔ 1/2H2 + OH- -0.83 Zn2+ + 2e- ↔ Zn -0.76 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ + 0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ + 7H2O +1.33 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged CI- ion CI2 gas (yellow gas) Discharged Ca2+ ion to Ca 2CI - ↔ CI2+ 2e Ca2+ + 2e ↔ Ca Compound broken down (LYSIS) energy needed О О Ca2+ CI - Eθ =-ve → supply +4.23v to breakdown CaCI2 → Ca+ CI2 Electrolysis (Molten Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Inert electrode Carbon/graphite CI - CI - CI - Ca2+ Ca2+ Ca2+
- 7. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion NaCI aqueous Electrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 4OH - ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O → 2H2 + O2 Eθ = -2.06V Eθ = -2.06V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Na+ , CI- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ + 0.77 Ag+ + e- ↔ Ag +0.80 O2 + 4H+ +4e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ +1.33 1/2CI2 + e- ↔ CI- +1.36 1/2F2 + e- ↔ F- +2.87 Discharged OH- ion O2 gas Discharged H+ ion to H2 gas О О Na+/H+ CI-/OH- Eθ =-ve → supply +2.06v to breakdown NaCI → H2 + O2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e Na+ + e ↔ Na Eθ = -2.71 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2CI- ↔ CI2 + 2e Eθ = -1.36 О Inert electrode Carbon/graphite OH- OH- CI - CI - H+ H+ Na+ Na+
- 8. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion NaI aqueousElectrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 2I - ↔ I2 + 2e Eθ = -0.54 NaI → H2 + I2 Eθ = -1.37V Eθ = -1.37V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Na+ , I- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged I- ion I2 Discharged H+ ion to H2 gas О О Na+/H+ I-/OH- Eθ = -ve → supply +1.37 v to breakdown NaI → H2 + I2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e Na+ + e ↔ Na Eθ = -2.71 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 О Oxidation Eθ > more +ve easier to lose e 2I- ↔ I2 + 2e Eθ = -0.54 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 О Inert electrode Carbon/graphite I - I - OH- OH- H+ H+ Na+ Na+
- 9. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion CuCI2 aqueous Electrolytic Cell Cu2+ + 2e ↔ Cu Eθ = +0.34 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 CuCI2 → Cu + O2 Eθ = -0.89V Eθ = -0.89V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Cu2+ , CI- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 Cu+ + e- ↔ Cu +0.52 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 1/2F2 + e- ↔ F- +2.87 Discharged OH- ion O2 Discharged Cu2+ ion to Cu metal О Cu2+/H+ CI-/OH- Eθ = -ve → supply +0.89 v to breakdown CuCI2 → Cu+ O2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Cu2+ + 2e ↔ Cu Eθ = +0.34 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2CI- ↔ CI2 + 2e Eθ = -1.36 ОО Inert electrode Carbon/graphite OH- OH- CI - CI - H+ H+ Cu2+ Cu2+
- 10. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion CuBr2 aqueousElectrolytic Cell Cu2+ + 2e ↔ Cu Eθ = +0.34 2Br- ↔ Br2 + 2e Eθ = -1.07 CuBr2 → Cu + Br2 Eθ = -0.73V Eθ = -0.73V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Cu2+ , Br- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 Cu+ + e- ↔ Cu +0.52 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged Br- ion Br2 Discharged Cu2+ ion to Cu О Cu2+/H+ Br-/OH- Eθ = -ve → supply +0.73 v to breakdown CuBr2 → Cu+ Br2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Cu2+ + 2e ↔ Cu Eθ = +0.34 О Oxidation Eθ > more +ve easier to lose e 2Br- ↔ Br2 + 2e Eθ = -1.07 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 Inert electrode Carbon/graphite Br- Br- OH- OH- Cu2+ Cu2+ H+ H+
- 11. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion KI aqueous Electrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 2I- ↔ I2 + 2e Eθ = -0.54 KI → H2+ Br2 Eθ = -1.37V Eθ = -1.37V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction K+ , I- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged I- ion I2 Discharged H+ ion to H2 О K+/H+ I-/OH- Eθ = -ve → supply +1.37 v to breakdown KI→ H2 + I2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e K+ + e ↔ K Eθ = -2.93 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 О Oxidation Eθ > more +ve easier to lose e 2I- ↔ I2 + 2e Eθ = -0.54 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 ОО Inert electrode Carbon/graphite OH- OH- I - I - H+ H+ K+ K+
- 12. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion K2SO4 aqueous Electrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 4OH- ↔ 2H2O+ O2 + 4e Eθ = -1.23 K2SO4 → H2+ O2 Eθ = -2.06V Eθ = -2.06V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction K+ , SO4 2- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 S2 O8 2- + 2e ↔ SO4 2- +2.01 1/2F2 + e- ↔ F- +2.87 Discharged OH- ion O2 Discharged H+ ion to H2 О K+/H+ SO4 2-/OH- Eθ = -ve → supply +2.06 v to breakdown K2SO4→ H2 + O2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e K+ + e ↔ K Eθ = -2.93 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2SO4 2- ↔ S2O8 2- + 2e Eθ = -2.01 ОО H2 gas Ratio 1:2 O2 gas Inert electrode Carbon/graphite OH- OH- SO4 2- SO4 2- K+ K+ H+ H+
- 13. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion AgNO3 aqueous Electrolytic Cell Ag+ + e ↔ Ag Eθ = +0.80 4OH- ↔ 2H2O+ O2 + 4e Eθ = -1.23 AgNO3 → Ag + O2 Eθ = -0.43V Eθ = -0.43V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction Ag+ , NO3 - + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 I2 + 2e- ↔ 2I- +0.54 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 S2 O8 2- + 2e ↔ SO4 2- +2.01 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged OH- ion O2 Discharged Ag+ ion to Ag О Ag+/H+ NO3 -/OH- Eθ = -ve → supply +0.43 v to breakdown AgNO3→ Ag + O2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Ag+ + e ↔ Ag Eθ = +0.80 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 NO3 - cannot be discharged Inert electrode Carbon/graphite OH- OH- NO3 - NO3 - H+ H+ Ag+ Ag+
- 14. Discharge of ions 1 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion H2SO4 aqueous Electrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 4OH - ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O → 2H2 + O2 Eθ = -2.06V Eθ = -2.06V -ve (NON spontaneous) Conversion electrical to chemical energy Energy needed to decompose compound!!!!!!!! Cation → Cathode (-ve) → Reduction H+ , SO4 2- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ + 0.77 Ag+ + e- ↔ Ag +0.80 O2 + 4H+ +4e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ +1.33 1/2CI2 + e- ↔ CI- +1.36 S2 O8 2- + 2e ↔ SO4 2- +2.01 1/2F2 + e- ↔ F- +2.87 Discharged OH- ion O2 gas Discharged H+ ion to H2 gas О О H+ SO4 2-/OH- Eθ =-ve → supply +2.06v to breakdown H2SO4 → H2 + O2 Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2SO4 2- ↔ S2O8 2- + 2e Eθ = -2.01 О H2 gas O2 gas Ratio 1:2 Inert electrode Carbon/graphite OH- OH- SO4 2- SO4 2- H+ H+ H+ H+
- 15. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion Conc NaCI Electrolytic Cell 2H+ + 2e ↔ H2 Eθ = -0.83 2CI - ↔ CI2 + 2e Eθ = -1.36 NaCI → 2H2 + CI2 + NaOH Eθ = -2.19 Cation → Cathode (-ve) → Reduction Na+ , CI- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 Cu+ + e- ↔ Cu +0.52 1/2I2 + e- ↔ I- +0.54 Fe3+ + e- ↔ Fe2+ + 0.77 Ag+ + e- ↔ Ag +0.80 O2 + 4H+ +4e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ +1.33 1/2CI2 + e- ↔ CI- +1.36 1/2F2 + e- ↔ F- +2.87 Discharged CI- ion CI2 gas Discharged H+ ion to H2 gas О О Na+/H+ CI-/OH- Inert electrode Carbon/graphite Eθ =-ve → supply +2.19v to breakdown NaCI → H2 + CI2 + NaOH Electrolysis (Concentrated Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e Na+ + e ↔ Na Eθ = -2.71 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2CI- ↔ CI2 + 2e Eθ = -1.36 О Ratio 1:2 H2 gas CI2 gas Dilute NaCI – OH- discharged due to Eθ value Conc NaCI – CI- discharged due to overpotential factor Discharged of H+ and OH- ion need addition voltage due to high activation energy for H2/O2 production If Conc CI- is high ↑ – it is preferred !!!!!! OH- OH- CI - CI - H+ H+ Na+ Na+
- 16. Discharge of ions 2 Cation + 2 Anion Oxidation ← Anode (+ve) ← Anion Conc CuCI2 Electrolytic Cell Cu2+ + 2e ↔ Cu Eθ = +0.34 2CI- ↔ CI2 + 2e Eθ = -1.36 CuCI2 → Cu + O2 Eθ = -0.89V Cation → Cathode (-ve) → Reduction Cu2+ , CI- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 Cu+ + e- ↔ Cu +0.52 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 1/2CI2 + e- ↔ CI- +1.36 1/2F2 + e- ↔ F- +2.87 Discharged CI- ion CI2 Discharged Cu2+ ion to Cu metal О Cu2+/H+ CI-/OH- Eθ = -ve → supply +0.89 v to breakdown CuCI2 → Cu+ O2 Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Cu2+ + 2e ↔ Cu Eθ = +0.34 О Oxidation Eθ > more +ve easier to lose e 4OH- ↔ 2H2O + O2 + 4e Eθ = -1.23 2H2O ↔ 4H+ + O2 + 4e Eθ = -1.23 2CI- ↔ CI2 + 2e Eθ = -1.36 ОО Inert electrode Carbon/graphite Electrolysis (Concentrated Salt) Dilute CuCI2 – OH- discharged due to Eθ value Conc CuCI2 – CI- discharged due to overpotential factor Discharged of H+ and OH- ion need addition voltage due to high activation energy for H2/O2 production If Conc CI- is high ↑ – it is preferred !!!!!! CI2 gas copper OH - OH - CI - CI - Cu2+ Cu2+ H+ H+
- 17. Carbon electrode Discharge of ions 2 Cation 2 Anion Oxidation ← Anode (+ve) ← Anion CuCI2 aqueous Electrolytic Cell Cation → Cathode (-ve) → Reduction Cu2+ , CI- + H+ , OH- (from water) + + + + + + - - - - - Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 Cu+ + e- ↔ Cu +0.52 I2 + 2e- ↔ 2I- +0.54 Fe3+ + e- ↔ Fe2+ +0.77 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ +1.33 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 Discharged Cu2+ ion to Cu metal О CI-/OH- Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Cu2+ + 2e ↔ Cu Eθ = +0.34 О Copper electrode as anode Cu easier discharge ↓ due nature electrode ↓ Cu → Cu2+ + 2e ↓ Cu electrode dissolve Copper electrode OH- discharged ↓ due to Eθ value ↓ 4OH- ↔ 2H2O+O2 + 4e ↓ O2 gas + + + + + Cu → Cu2+ + 2e copper electrode Cu → 2e + Cu2+ Cu2+ Cu2+ Cu2+ Cu2+ Cu → 2e + Cu2+ Cu → 2e + Cu2+ Cu2+ Cu2+ e- e- e e e- e- e - At Anode Copper electrode oxidizes/dissolve Conc copper ions unchanged Mass of Cu anode decreased Mass of Cu cathode increased Cu2+ Cu2+ Cu2+ OH- OH- CI - CI - H+ H+ Cu2+ Cu2+ Cu2+/H+
- 18. AgNO3 aqueous Electrolytic Cell Carbon electrode Discharge of ions 2 Anion Oxidation ← Anode (+ve) ← Anion Cation → Cathode (-ve) → Reduction Ag+ , NO3 - + H+ , OH- (from water) + + + + + + - - - - - NO3 -/OH- Electrolysis (Aqueous Salt) Factor affecting ion discharged (Selective Discharge) ↓ - Molten/aqueous - Relative E values of ion - Conc ion – conc/diluted - Nature of electrode Reduction Eθ > more +ve easier gain e 2H+ + 2e ↔ H2 Eθ = -0.83 2H2O +2e- ↔ H2 + 2OH- Eθ = -0.83 Ag+ + e ↔ Ag Eθ = +0.80 Copper electrode as anode Ag easier discharge ↓ due nature electrode ↓ Ag → Ag+ + e ↓ Ag electrode dissolve Silver electrode OH- discharged ↓ due to Eθ value ↓ 4OH- ↔ 2H2O+O2 + 4e ↓ O2 gas + + + + + Ag → Ag+ + e silver electrode Ag → e + Ag+ Ag+ Ag+ Ag+ Ag+ Ag → e + Ag+ Ag → e + Ag+ Ag+ Ag+ e- e- e e e- e- e - At Anode Silver electrode oxidizes/dissolve Conc silver ions unchanged Mass of Ag anode decreased Mass of Ag cathode increased Ag+ Ag+ Ag+ Oxidized sp ↔ Reduced sp Eθ/V Li+ + e- ↔ Li -3.04 K+ + e- ↔ K -2.93 Ca2+ + 2e- ↔ Ca -2.87 Na+ + e- ↔ Na -2.71 Mg 2+ + 2e- ↔ Mg -2.37 Al3+ + 3e- ↔ AI -1.66 Mn2+ + 2e- ↔ Mn -1.19 2H2O +2e- ↔ H2 + 2OH- -0.83 Fe2+ + 2e- ↔ Fe -0.45 Ni2+ + 2e- ↔ Ni -0.26 Sn2+ + 2e- ↔ Sn -0.14 Pb2+ + 2e- ↔ Pb -0.13 H+ + e- ↔ 1/2H2 0.00 Cu2+ + e- ↔ Cu+ +0.15 SO4 2- + 4H+ + 2e- ↔ H2SO3 + H2O +0.17 Cu2+ + 2e- ↔ Cu +0.34 1/2O2 + H2O +2e- ↔ 2OH- +0.40 I2 + 2e- ↔ 2I- +0.54 Ag+ + e- ↔ Ag +0.80 1/2Br2 + e- ↔ Br- +1.07 O2 + 4H+ +4e- ↔ H2O +1.23 Cr2O7 2-+14H+ +6e- ↔ 2Cr3+ +1.33 1/2CI2 + e- ↔ CI- +1.36 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 S2 O8 2- + 2e ↔ SO4 2- +2.01 MnO4 - + 8H+ + 5e- ↔ Mn2+ + 4H2O +1.51 1/2F2 + e- ↔ F- +2.87 ОО Discharged Ag+ ion to Ag - - - - - OH - OH - NO3 - NO3 - Ag+ Ag+ H+ H+ Ag+/H+
- 19. Electrolyte Electrode Ions Cathode (-) Anode (+) PbBr2 (molten) Carbon Pb2+/ Br- Pb2+ + 2e → Pb Pb 2Br- → Br2 + 2e Br2 CaCI2 (molten) Carbon Ca2+ /CI- Ca2+ +2e → Ca Ca 2CI- → CI2 + 2e CI2 NaCI Carbon Na+/ CI– H+/OH- 2H+ + 2e → H2 H2 4OH- ↔ 2H2O +O2 + 4e O2 NaCI (conc) Carbon Na+/ CI– H+/OH- 2H+ + 2e → H2 H2 2CI- → CI2 + 2e CI2 NaI Carbon Na+/ I– H+/OH- 2H+ + 2e → H2 H2 2I- → I2 + 2e I2 CuCI2 Carbon Cu2+/ CI– H+/OH- 2H+ + 2e → H2 H2 4OH- ↔ 2H2O +O2 + 4e O2 CuCI2 (conc) Carbon Cu2+/CI- H+/OH - 2H+ + 2e → H2 H2 2CI- → CI2 + 2e CI2 CuCI2 Copper Cu2+/CI- Cu2++2e → Cu Cu Cu → Cu2++ 2e Cu CuBr2 Carbon Cu2+/Br- H+/OH - 2H+ + 2e → H2 H2 2Br- → Br2 + 2e Br2 KI Carbon K+/I- H+/OH - 2H+ + 2e → H2 H2 2I- → I2 + 2e I2 AgNO3 Carbon Ag+/NO3 - H+/OH - Ag+ + e → Ag Ag 4OH- ↔ 2H2O +O2 + 4e O2 AgNO3 Silver Ag+/NO3 - Ag+ + e → Ag Ag → Ag+ + e K2SO4 Carbon K+/SO4 2- H+/OH - 2H+ + 2e → H2 H2 4OH- ↔ 2H2O +O2 + 4e O2 H2SO4 Carbon H+/SO4 2- H+/OH - 2H+ + 2e → H2 H2 4OH- ↔ 2H2O +O2 + 4e O2 HCI Carbon H+/CI- H+/OH - 2H+ + 2e → H2 H2 4OH- ↔ 2H2O +O2 + 4e O2 HCI (conc) Carbon H+/CI- H+/OH - 2H+ + 2e → H2 H2 2CI- → CI2 + 2e CI2 Ease Anion discharged NO3 – SO4 2- CI– Br– I– OH– Ease Cation discharged K+ Ca2+ Na+ Mg2+ Al 3+ Zn2+ Fe2+ Sn2+ Pb2+ H+ Cu2+ Ag+ easier easier Electrolyticcell Conversion electrical to chemical energy + - Anode (+ve) Oxidation Cathode (-ve) Reduction CathodeAnode Factor affecting ion discharged (Selective Discharge) Relative E values of ion Conc ion conc/diluted Nature of electrode PANIC Positive is Anode, Negative Is Cathode NO3 – - diff to discharge - ON for N is +5 (very high) - Diffto lose e to get higher
- 20. Current– measured in Amperes or Coulombs per second 1A = 1 Coulomb charge pass througha point in 1 second = 1C/s 1 Coulomb charge (electron)= 6.28 x 10 18 electronspassing in 1 second 1 electron - carry charge of – 1.6 x 10 -19 C 6.28 x 10 18 electron - carry charge of - 1 C 1A 6.02 x 10 23 electron (1 Mol) - carry charge of - 96500C 1F Electriccurrent Flow electric charges(electron) From High electric potential– low potential ond electron ond Coulomb A sec.1 .1028.6 sec1 1 1 18 Current Flow of charges - - - ItQ t = Time/ s Find amt charges pass through a sol if Current is 2.ooA, time is 15 mins ItQ Q = Amt Charges/ C I = Current/ A CQ 1800601500.2 Faraday’s constant (F) – charge on 1 mol of electron 96500 C mol-1 1 1923 965001 106.11002.6 CmolF CF eLF 1A = 6.28 x 1018 e 1 second L = Avogadro constant 1 Faraday – Quantity charge 96500C supply to 1 mol electron Faraday's 1st Law Electrolysis Faraday's 2nd Law Electrolysis Amt charges (Q) Mass produce is directly proportional to the quantity of electricity/charges ( C ) Factor affecting mass substance liberated Chargeon ion Current Time ItQ Mass produce is inversely proportional to charges on ion Cu2+ + 2e ↔ CuAg+ + e ↔ Ag AI3+ + 3e ↔ AI +1 +2 +3 1 mol e → 1 mol Ag 2 mol e → 1 mol Cu 3 mol e → 1 mol AI Pass 1 mol e 1 mol e → 1 mol Ag 1 mol e → 1/2 mol Cu 1 mol e → 1/3 mol AI
- 21. Current– measured in Amperes or Coulombs per second 1A = 1 Coulomb charge pass througha point in 1 second = 1C/s 1 Coulomb charge (electron)= 6.28 x 10 18 electronspassing in 1 second 1 electron - carry charge of – 1.6 x 10 -19 C 6.28 x 10 18 electron - carry charge of - 1 C 1A 6.02 x 10 23 electron (1 Mol) - carry charge of - 96500C 1F Electriccurrent Flow electric charges(electron) From High electric potential– low potential ond electron ond Coulomb A sec.1 .1028.6 sec1 1 1 18 Current Flow of charges - - - ItQ t = Time/ s Find amt charges pass through a sol if Current is 2.ooA, time is 15 mins ItQ Q = Amt Charges/ C I = Current/ A CQ 1800601500.2 Faraday’s constant (F) – charge on 1 mol of electron 96500 C mol-1 1 1923 965001 106.11002.6 CmolF CF eLF 1A = 6.28 x 1018 e 1 second L = Avogadro constant 1 Faraday – Quantity charge 96500C supply to 1 mol electron Copper (II) sulfate electrolyzed using current -- 0.150A for 5 hrs. Cal mass of Cu deposited CQ Q ItQ 2700 60605150.0 Cu2+ + 2e ↔ Cu 2 mol e → 1 mol Cu 0.028 mol e → 0.014 mol Cu emolC emolC ...028.0 96500 2700 2700 ...196500 Find Current/I → Find Charge/Q → Find mol electron → Find Mass deposited use Faraday’s constant Mass = mol x RAM Mass = 0.014 x 63.5 Mass = 0.889 g Mass deposited (Cathode) Cu 1 Cu2+ Cu2+
- 22. Electrolysis AI t Q I ItQ 4.6 605.12 4787 Cr3+ + 3e ↔ Cr 1 mol Cr → 3 mol e 0.0165 mol Cr → 0.0495 mol e Find Mass → Find mol electron → Find Charges/Q → Find current/I use Faraday’s constant Mass = mol x RAM 0.86 = mol x 52.00 mol = 0.0165 Electrolysis Cr2(SO4)3 yield 0.86g of Cr after passing current for 12.5 min. Find amt of current used. 1 mol e → 96500C 0.0495mol e → 96500 x 0.0495 = 4787 C Find time /hrs need to produce 25g of Cr from Cr2(SO4)3 with current of 1.1A Find Mass → Find mol electron → Find Charges/Q →Find current/I Cr3+ + 3e ↔ Cr use Faraday’s constant 1 mol Cr → 3 mol e 0.48 mol Cr → 1.44 mol e Mass = mol x RAM 25 = mol x 52.00 mol = 0.48 1 mol e → 96500C 1.44mol e → 96500 x 1.44 = 138960C 1.35 1.1 138960 t I Q t ItQ Mass deposited (Cathode) Cr3+ Cr3+ Cr Find vol of H2 gas collect at cathode when aq sol Na2SO4 electrolyzed for 2.00 hours with a 10A. Mass deposited (Cathode) Cr Cr3+ Cr3+ Find Current/I → Find Charge/Q → Find mol electron → Find Vol 2H+ + 2e ↔ H2 CQ Q ItQ 72000 6060200.2 use Faraday’s constant emolC emolC ...746.0 96500 72000 72000 ...196500 2 mol e → 1 mol H2 0.746 mol e → 0.373 mol H2 H2 O2 2 3 4 Vol = 8.35 dm3
- 23. Faraday's 1st Law Electrolysis Faraday's 2nd Law Electrolysis Amt charges (Q) Mass produce is directly proportional to the quantity of electricity/charges ( C ) Factor affecting mass substance liberated Chargeon ion Current Time ItQ Mass produce is inversely proportional to charges onion Cu2+ + 2e ↔ CuAg+ + e ↔ Ag AI3+ + 3e ↔ AI +1 +2 +3 1 mol e → 1 mol Ag 2 mol e → 1 mol Cu 3 mol e → 1 mol AI Pass 1 mol electron across 1 mol e → 1 mol Ag 1 mol e → 1/2 mol Cu 1 mol e → 1/3 mol AI Ag+ Ag+ - - - - - - + + + + + + Cu2+ Cu2+ AI3+ AI3+ AgNO3,CuSO4, AICI3 connect in series. Same amt current used. Cal mass Cu and Al when 10.8 g Ag deposited. Ag+ + e ↔ Ag 1 mol Ag → 1 mol e 0.1 mol Ag →0.1 mol e Mass = mol x RAM 10.8 = mol x 108 mol = 0.1 Cu2+ + 2e ↔ Cu 2 mol e → 1 mol Cu 0.1 mol e → 0.05 mol Cu AI3+ + 3e ↔ AI 3 mol e → 1 mol AI 0.1 mol e → 0.03 mol AI Mass Cu = 0.05 mol Mass AI = 0.03 mol AgNO3, H3SO4 connect in series. Same amt current used Cal vol H2,O2 when 10.8 g Ag deposited. - - Ag+ Ag+ O2 H2 Ag+ + e ↔ Ag 1 mol Ag → 1 mol e 0.1 mol Ag → 0.1 mol e Mass = mol x RAM 10.8 = mol x 108 mol = 0.1 2H+ + 2e ↔ H2 2 mol e → 1 mol H2 0.1 mol e → 0.05 mol H2 4OH- ↔ 2H2O +O2 + 4e 4 mol e → 1 mol O2 0.1 mol e → 0.025 mol O2 2.24 dm3 0.56 dm3
- 24. Faraday's 1st Law Electrolysis Faraday's 2nd Law Electrolysis Amt charges (Q) Mass produce is directly proportional to the quantity of electricity/charges ( C ) Factor affecting mass substance liberated Chargeon ion Current Time ItQ Mass produce is inversely proportional to charges onion Cu2+ + 2e ↔ CuAg+ + e ↔ Ag AI3+ + 3e ↔ AI +1 +2 +3 1 mol e → 1 mol Ag 2 mol e → 1 mol Cu 3 mol e → 1 mol AI Pass 1 mol electron across 1 mol e → 1 mol Ag 1 mol e → 1/2 mol Cu 1 mol e → 1/3 mol AI Purification of metal Applicationof Electrolysis Extractionreactivemetal Aluminium Sodium - ve electrode Aluminium metal AI2O3 Al3+ + 3e → Al Electroplating - Prevent corrosion - Improve appearance Copper, chromium,silver - ve Sodium metal Na+ + e → Na NaCI + ve - - - - - - - - + + + + + + + + + + + + Anode (+ve) Plating metal Cathode (-ve) Object + + - - Anode (+ve) Impure Cu metal Mass decrease Cathode (-ve) Pure Cu metal Mass increase Cu2+ + 2e ↔ Cu Cu2+ Cu2+ Cu2+ Cu ↔ Cu2+ + 2e 2CI- -2e → CI2
- 25. Electrolysis of KI Electrolysis of waterExcellent Silver crystalformation Galvanizing Iron with Zinc PANIC Positive is Anode, Negative Is Cathode Factor affecting ion discharged (Selective Discharge) Relative E values of ion Conc ion conc/diluted Nature of electrode Ease Cation discharged K+ Ca2+ Na+ Mg2+ Al 3+ Zn2+ Fe2+ Sn2+ Pb2+ H+ Cu2+ Ag+ easier Ease Anion discharged NO3 – SO4 2- CI– Br– I– OH– easier NO3 – - diff to discharge - ON for N is +5 (very high) - Diffto lose e to get higher Anode (+ve) Oxidation Cathode (-ve) Reduction Conversion electrical to chemical energy Electrolyticcell + - Faraday's 1st Law Electrolysis Mass produce is directly proportional to the quantity of electricity/charges ( C ) Factor affecting mass substance liberated Amt charges (Q) Chargeon ion Current Time ItQ Faraday's 2nd Law Electrolysis Mass produce is inversely proportional to charges on ion +1 +2 Ag+ + e ↔ Ag Cu2+ + 2e ↔ Cu 1 mol e → 1 mol Ag 2 mol e → 1 mol Cu 1 mol e → 1 mol Ag 1 mol e → 1/2 mol Cu Pass 1 mol electron across
