2 22 What Is The Combined Gas Law
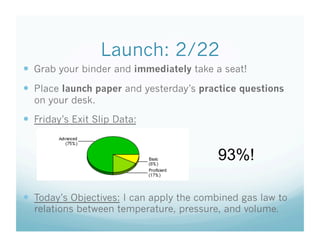
- 1. Launch: 2/22 Grab your binder and immediately take a seat! Place launch paper and yesterday’s practice questions on your desk. Friday’s Exit Slip Data: 93%! Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume.
- 2. Launch: 2/22 Grab your binder and immediately take a seat! Place launch paper and yesterday’s practice questions on your desk. Friday’s Exit Slip Data: 88% Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume.
- 3. Launch: 2/22 Grab your binder and immediately take a seat! Place launch paper and yesterday’s practice questions on your desk. Friday’s Exit Slip Data: 97%! Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume.
- 4. Launch: 2/22 Grab your binder and immediately take a seat! Place launch paper and yesterday’s practice questions on your desk. Friday’s Exit Slip Data: 95%! Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume.
- 5. Launch 2/12 1. Turn to your partner and ask them: 1. What was the most interesting thing that you did over break?
- 6. Launch 2/10 2. Using your notes, which pairs of variables (P V, & T) are directly related? ,
- 7. Launch 2/10 3. Using your notes, write down the three equations that we learned that show relationships between P V, &T. ,
- 8. Announcements Welcome back! Exam on Thursday! One day of new material Two days of review – Jeopardy? This week we need to be efficient Follow our 7 class rules Learn it now! – ask questions Class points start over today!
- 9. February Calendar Quiz! Today Exam!
- 10. Opening Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume. Standard – CH.4.c Today’s Questions: What is the combined gas law? Simulation!
- 11. What is the combined gas law? Mr. Heffner 2/22/10
- 12. Review: What are P T, and V? , Temperature (T) measures… movement in units of K & °C Pressure (P) measures… collisions in units of atm & mmHg Volume (V) measures… space in units of L & mL
- 13. Review: How are P V, and T related? , P&T V&T P&V T= P T= V V= P directly related directly related indirectly related P1 P2 V1 V2 = T = T P1× V1=P2× V2 T1 2 T1 2 d = division Gas Laws
- 14. What is the combined gas law? The combined gas law is… P1× V1 P2× V2 = T1 T2
- 15. How are P & T related? There is a 3 step process for solving gas equations: P1 × V1 P2 × V2 1. Write down = T1 T2 2. Circle the given units and the letters that go with them Cross out the letter that is “fixed”, “constant”, or “same” 3. Re-write the equation, plug-in, and solve!
- 16. Example #1 At constant volume, a gas at 100K and 1 atm is heated to 300K. What is the new gas pressure? Step 1: Write down the combined gas law P1× V1 P2× V2 = T1 T2
- 17. Example #1 At constant volume, a gas at 100K and 1 atm is heated to 300K. What is the new gas pressure? Step 2: Circle the given units and letters that belong to them. Cross out the letter that is “fixed”, “constant”, or “same”. P1× V1 P2× V2 = T1 T2
- 18. Example #1 At constant volume, a gas at 100K and 1 atm is heated to 300K. What is the new gas pressure? Step 3: Re-write the equation, plug-in, and solve!. cross- P1 1atm P2 multiply = T ✕ 300K T1 100K 2 (P2)(100K) = (1 atm)(300K) 100K 100K P2 = 3 atm
- 19. Example #2 The temperature of a gas is 50°C in a container with a fixed pressure. If the initial volume of the gas is 200mL, what would the volume be if the sample was cooled to 25°C? Step 1: Write down the combined gas law P1× V1 P2× V2 = T1 T2
- 20. Example #2 The temperature of a gas is 50°C in a container with a fixed pressure. If the initial volume of the gas is 200mL, what would the volume be if the sample was cooled to 25°C? Step 2: Circle the given units and letters that belong to them. Cross out the letter that is “fixed”, “constant”, or “same”. P1× V1 P2× V2 = T1 T2
- 21. Example #2 The temperature of a gas is 50°C in a container with a fixed pressure. If the initial volume of the gas is 200mL, what would the volume be if the sample was cooled to 25°C? Step 3: Re-write the equation, plug-in, and solve! V1 200mL V2 cross- multiply = T ✕ 25°C T1 50°C 2 (V2)(50°C) =(200mL)(25°C) 50°C 50°C V2 = 100 mL
- 22. Example #3 The volume of 200L of Cl2 gas at 200mmHg is decreased to 100L. What is the new gas pressure? Step 1: Write down the combined gas law P1× V1 P2× V2 = T1 T2
- 23. Whiteboards Work in pairs Trade-off marker every question You have 60 seconds to calculate the answer Follow the steps! 1. Write down the equation 2. Circle and cross out 3. Plug-in and solve! Don’t forget units! Lift board only when prompted
- 24. At constant temperature, a sample of CO2 gas occupies a volume of 10L at 5 atm. What will the volume of the gas be if the pressure is increased to 2 atm?
- 25. The volume of Ne gas is 25L at 50K at a fixed pressure. If the volume is increased to 100L, what would the new temperature be?
- 26. The pressure of He gas is 100mmHg at 80K. If the temperature is reduced to 60K, what would the new pressure be?
- 27. A sample of CO2 gas occupies a volume of 1L at 200mmHg. What will the volume of the gas be if the pressure is decreased to 100mmHg under constant temperature?
- 28. Practice Questions Practice question worksheet
- 29. Closing Today’s Objectives: I can apply the combined gas law to relations between temperature, pressure, and volume. Standard – CH.4.c Today’s Questions: What is the combined gas law? Simulation!
- 30. Exit Slip 1. Which of the following is the combined gas law? a. V2 V1 c. T1× V1 T2× V2 = T = T1 2 P1 P2 b. d. P1× V1 P2× V2 P1× V1 = P2× V2 = T1 T2
- 31. Exit Slip 2. Which of the following is a correct statement? a. mL is a unit of pressure b. mL is a unit of volume, K is a unit of temperature, and mmHg is a unit of pressure c. mmHg is a unit of volume, K is a unit of temperature, and L is a unit of pressure d. K and L are both units of temperature
- 32. Exit Slip 3. Choose the right equation. At a constant temperature, a sample of CO2 gas occupies a volume of 20mL at 2 atm. What will the volume of the gas be if the pressure is increased to 20 atm? a. P1 P2 c. = V T1× V1 = T2× V2 V1 2 b. d. V1 V2 P1× V1 = P2× V2 = T T1 2
- 33. Exit Slip 4. The volume of 400mL of Ne gas at 100mmHg is decreased to 200mL at constant temperature. What is the new gas pressure? a. .5 mmHg b. 200 mmHg c. 200 mL d. 2000 mmHg
- 34. Exit Slip 5. How are P & T related? a. They are indirectly related. b. An decrease in temperature increases pressure. c. They change in the opposite direction d. They are directly related
- 35. Homework Finish practice questions