C3d The Group 1 Elements
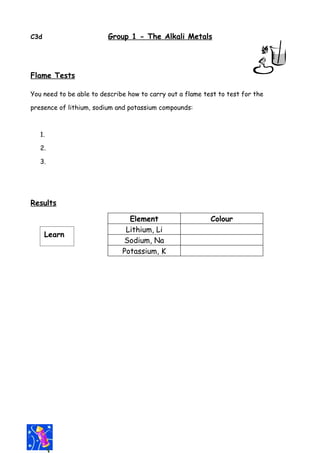
- 1. C3d Group 1 - The Alkali Metals Flame Tests You need to be able to describe how to carry out a flame test to test for the presence of lithium, sodium and potassium compounds: 1. 2. 3. Results Element Colour Lithium, Li Learn Sodium, Na Potassium, K
- 2. C3d Group 1 - The Alkali Metals In the table is some information about lithium sodium and potassium. Use this information to predict the properties of Rubidium, Caesium and Francium: Name Appearance Flame Colour Denisty Melting Boiling point (used in fireworks ! ) gcm-3 point ( 0C ) ( 0C ) Lithium Shiny, soft, silver- red 0.53 181 1342 Li grey Sodium Shiny, soft, silver- yellow 0.97 98 883 Na grey Potassium Shiny, soft, silver- lilac 0.86 63 760 K grey Rubidium Soft, silvery-white red Rb Caesium Cs Soft, shiny, gold- blue coloured, Francium Not available in visible unknown Fr quantities Trends (patterns): • Group 1 metals are all _______ • They are generally ___________ and ____________ in colour • Their density generally ________________ as you go down the group. The first __ have a lower density than water (1.0gcm-3) so they _________ on it. • All of group 1 are very _____________ metals. They can be easily cut with a knife. They get ________________ as you go down the group. Why are they called the Alkali Metals?
- 3. For this we have to look at their reactivity with water. Watch the demonstration and then fill in the sheet. Alkali Observations (What did you see happening) Colour of universal metal indicator Li • floats • fizzes __________, disappears __________ • moves _______ around the surface (as a cube) Na • floats • fizzes __________, disappears __________ • melts to form a ball • moves _____________ over the surface K • floats • fizzes and disappears _________________ • ________ to form a ball • moves ________________ over the surface • burns with a ____________ flame LEARN • The gas produced is ______________. • Test for hydrogen: a _________________ • We can see from the colour of the universal indicator (blue/purple) that an Result: it burns with a “_______________” alkaline solution is produced when the alkali metals are reacted with water. This is why Group 1 is called the Alkali Metals.
- 4. • The alkali metals react __________________ with water so they are stored under ______ to stop them reacting with _______ and ________________. • the reactivity with water increases down Group 1 • an alkali is formed which is the hydroxide of the metal What reaction is taking place? lithium + water lithium hydroxide + hydrogen sodium + water + potassium + water + Predict how rubidium would react with water, in terms of what you would observe happening and the products that would be made. Write equations. Notice that all the equations are balanced in exactly the same way, it’s only the symbol of the metal that changes. This means that you only need to learn 1 word and 1 symbol equation !! all of group 1 react with water in this way? Why do • All of the Group 1 metals have _______ electron in the outer shell.
- 5. • All of them want to react and ____ that one electron because this leaves them with a full shell underneath which is a more ___________ electronic structure to have. • It is relatively easy to lose just one electron so they are all very __________ metals. • The more reactive the alkali metal, the easier it is for an atom to lose one electron and form a ________________ ion. Oxidation (and reduction) When an atom loses an electron it becomes a positive ion. We can show this in a special type of equation called an “_______________________” e.g. • When an atom or ion loses electrons we is has been __________________ or that is it an oxidation reaction. • If an atom or ion gains electrons we say it has been ____________________ or it is a reduction reaction OIL RIG Oxidation Is Loss of electrons Reduction Is Gain of electrons Task: Write equations to show how sodium and potassium form ions Sodium: Potassium: