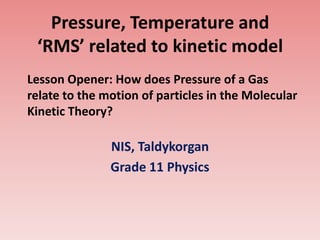
Pressure, temperature and ‘rms’ related to kinetic model
•Als PPTX, PDF herunterladen•
6 gefällt mir•18,347 views
The macroscopic properties of a gas, pressure and temperature, are explained in terms of molecule movement of the Kinetic Theory. The derivation of formulas are shown in logical steps for pressure, temperature and KE.
Melden
Teilen
Melden
Teilen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Experiment 9: Molecular Weight Determination using Freezing Point Depression

Experiment 9: Molecular Weight Determination using Freezing Point Depression
Ähnlich wie Pressure, temperature and ‘rms’ related to kinetic model
Ähnlich wie Pressure, temperature and ‘rms’ related to kinetic model (20)
Kinetic Molecular Theory solids, liquids and gases

Kinetic Molecular Theory solids, liquids and gases
Class 11 Physics Revision Notes Kinetic Theory.pdf

Class 11 Physics Revision Notes Kinetic Theory.pdf
Chem II - Kinetic Molecular Theory of Gases (Liquids and Solids)

Chem II - Kinetic Molecular Theory of Gases (Liquids and Solids)
PHYSICS CLASS XII Chapter 3 - Kinetic theory of gases and radiation

PHYSICS CLASS XII Chapter 3 - Kinetic theory of gases and radiation
CLASS XII PHYSICS Chapter 13 - Kinetic theory of gases and radiation

CLASS XII PHYSICS Chapter 13 - Kinetic theory of gases and radiation
Rise and fall of the clockwork universe - matter in extremes r2 OCR Physics B

Rise and fall of the clockwork universe - matter in extremes r2 OCR Physics B
Mehr von Michael Marty
Mehr von Michael Marty (8)
Future Energy Russian Будущий энергетический потенциал Казахстана(1)

Future Energy Russian Будущий энергетический потенциал Казахстана(1)
Resonance and natural frequency, uses and precautions nis

Resonance and natural frequency, uses and precautions nis
Introduction to oscillations and simple harmonic motion

Introduction to oscillations and simple harmonic motion
Accelerated expansion of universe and evolving ideas about gravity

Accelerated expansion of universe and evolving ideas about gravity
Intro to astrophysics nis grade 11 by mr marty, visible brightness = apparent...

Intro to astrophysics nis grade 11 by mr marty, visible brightness = apparent...
Kürzlich hochgeladen
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Kürzlich hochgeladen (20)
Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...

Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Beyond the EU: DORA and NIS 2 Directive's Global Impact

Beyond the EU: DORA and NIS 2 Directive's Global Impact
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Pressure, temperature and ‘rms’ related to kinetic model
- 1. Pressure, Temperature and ‘RMS’ related to kinetic model Lesson Opener: How does Pressure of a Gas relate to the motion of particles in the Molecular Kinetic Theory? NIS, Taldykorgan Grade 11 Physics
- 2. Objective 1: • Explain how molecular movement causes the pressure exerted by a gas and hence deduce the relationship: – Kazakh /Russian p=pressure n=molecular density m0=mass of a molecule <v>2= average velocity IB, SAT and Giancoli P=pressure N=Number of molecules m=mass of a molecule V=volume =average velocity
- 3. WHAT CAUSES PRESSURE in a GAS? IF MOLECULES MOVE FASTER AND COLLIDE MORE OFTEN, THEY HAVE MORE FORCE. PRESSURE INCREASES! When you shack a bottle of soda, the gas molecules move faster, pressure increases, when opened the higher pressure ‘explodes’ with the soda making a big mess.
- 4. How Does KT explain Pressure? Elastic collisions causes a force on the sides of the container. The force depends on: •Mass of molecules •Velocity of molecules •Number of molecules N=Number of molecules m=mass of a molecule =average velocity
- 5. Deduce (or Derive) this relationship… One molecule creates a force by its change in momentum F= Change in momentum change in time F = Δmvx = mvx-(-mvx) =2mvx Δt Δt 2L/vx F = mvx2 P = F = mvx2 /L L A L2 P= mvx2 = mvx2 This Pressure for L3 V for one molecule 2L= vxΔt → Δt = 2L/vx
- 6. The Pressure for N molecules and ‘mean square velocity’ • N molecules move at an average velocity = N • Velocity is a vector so In Russian/Kazakh ‘n’ = N/V and is called molecular density. So the Course Plan Formula is and • So P = 1 Nm 3V ‘n’ = N V (only here does ‘n’= molecular density)
- 7. YouTube Presentation of Pressure • Derivation of Pressure Formula: http://www.youtube.com/watch?v=CmerWVk 0ZaI
- 8. Root Mean Square Speed • Objective 2: Recall and understand the term root mean-square velocity (average = mean) • Simply – ‘the square root of the average velocity’ • Why? Velocities are + and - , so an average would be zero! • Squaring first gives all positive velocities • Taking the square give the average speed without direction. Also used in AC current calculations in year 12!
- 9. Why and Where do we need RMS? To find the velocity of molecules at T! • Start with → • Remember: PV = NkT • Therefore: → • Now, to find velocity we need to take the square root of the ‘average square velocity’!
- 10. Uses of RMS Velocity • RMS speed is used to predict how fast molecules are moving at a given Temperature. How fast molecules move is directly proportional to their absolute temperature and inversely proportional to their mass. vrms 3RT M Because:
- 11. Example: Determine the Speed of Molecules in Air at 293 K? N2 molecules (molecular mass 28.0 u) 3RT O2 molecules (molecular mass 32.0 u) vrms M For nitrogen… 3RT vrms M 3 8.31J mol K 293 K 0.0280 kg mol 511 m s 3 8.31J mol K 293 K 0.0320 kg mol 478 m s For oxygen… vrms 3RT M
- 12. At the same temperature… • Small molecules move fast • Large molecules move slow
- 13. Important features of Maxwell-Boltzmann distribution : The speed-density relationship of 4 Gases at 298.15 K (25 °C).
- 14. Kinetic Energy and Temperature: • Objective 3: “compare and PV=NkT and hence deduce that the average kinetic energy of a molecule is proportional to T” ! • Remember that • Therefore and
- 15. References: • Learn Physics: Learn about Kinetic Theory of Gases (good animation) http://www.youtube.com/watch?v=YSTRa27a3BQ • Pressure and RMS Velocity (Easy Formulas) http://www.youtube.com/watch?v=CmerWVk0ZaI
