India medical device regulatory process
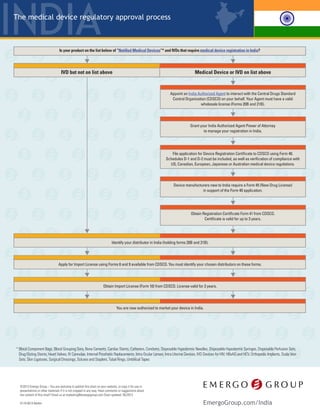
- 1. India © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 09/2016. EmergoGroup.com/India Medical devices and IVDs are regulated by the Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO); part of the Ministry of Health and Family Welfare. The regulatory framework for medical devices is based on drug regulations under the Drugs and Cosmetics Act of 1940, and Drugs and Cosmetics Rules of 1945. • Ablation Devices† • Blood Component Bags • Blood Grouping Sera • Bone Cements • Cardiac Stents • Catheters • Condoms • Dental Implants† • Disposable Hypodermic Needles • Disposable Hypodermic Syringes • Disposable Perfusion Sets • Drug Eluting Stents • Heart Valves • HBsAG and HCV • IV Cannulae • IVD Devices for HIV • Internal Prosthetic Replacements • Intra Ocular Lenses • Intra Uterine Devices • Orthopedic Implants • Scalp Vein Sets • Skin Ligatures • Surgical Dressings • Sutures and Staplers • Tubal Rings • Umbilical Tapes Currently only a limited number of medical devices and IVDs require registration in India.† They are: Appoint an India Authorized Agent to interact with the CDSCO on your behalf. Your Agent must have a valid wholesale license (Forms 20B and 21B/21C), and be granted Power of Attorney to manage your registration in India. Compile device application (Form 40), including: manufacturing facility information, device technical information, ISO 13485 certificate, IFU, testing results (if applicable), clinical data (if applicable), proof of approval in: the US, EU, Australia, Canada, or Japan, plus proof of approval in your home country (satisfied by CFS/CFG). File registration applications with the CDSCO. Pay fees. All documents must be in English. The CDSCO will issue a Registration Certificate (Form 41), which is valid for 3 years. The CDSCO reviews applications and may require a Technical Presentation.** Novel devices will also undergo a Subject Expert Committee (SEC) review.*** Notified IVDs require in-country performance testing through the National Institute of Biologicals (NIB). Seven types of non-notified IVDs and certain products for testing blood glucose require in-country performance testing. Results will be submitted with your import license application.* Non-notified IVD (NOT on list above) Notified Medical Device or IVD (On list above) Identify your distributor in India and apply for Import License. Qualified distributors must hold Forms 20B and 21B/21C. Obtain Import License (Form 10) from the CDSCO. The Import License will be issued in the name of your distributor. You are now authorized to market your device or IVD in India. 5119-0916 Regulatory Process for Medical Devices and IVDs † Some products that do not appear on the official list still require registration. Ablation devices, dental implants, and hernia mesh are examples. All IVDs, including Notified and Non-Notified, require an Import License. Medical devices which do not require registration have no restrictions and may be imported directly. Please contact us for details. * The seven types of non-notified IVDs requiring performance testing are: malaria, dengue, chikungunya, syphilis, typhoid, tuberculosis, and cancer markers. Testing must be conducted by a local accredited test laboratory. Blood glucose test strips and fully automated analyser based glucose reagents require performance testing from NIB (NIB also tests Notified IVDs). ** Approximately 25% of applications require a formal Technical Presentation. The Technical Presentation is an in-person meeting with the CDSCO to discuss the product in more detail. A representative from the manufacturer (such as an engineer) is expected to attend this meeting along with the India Authorized Agent. *** Devices novel to the Indian market (new technology, material, intended use), may face additional regulatory hurdles. CDSCO may require clinical studies conducted in India prior to regulatory approval, or the agency may issue a restricted approval. A restricted approval could include a requirement to actively collect and submit post-market data. The SEC meeting will include local clinicians and other experts who will weigh in on the acceptability of the existing clinical data. This is a simplified overview of the process. The CDSCO may choose to audit your submission and request more documents, which will add time to your approval.
- 2. India Notes 1. The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. 2. Registrations remain valid for the time specified as long as you do not make changes to the device, intended use or indications for use. 3. We recommend starting the re-registration process no later than the time period specified above. However, please consult with your distributor or regulatory expert well before this suggested time to avoid any lapse in your registration. 4. Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country. The European CE Marking process is considered the mid-point to which all other markets are compared. 5. Low = Less than US$5000; Midpoint = US$15000-$30000: High = More than US$50000. Overall cost includes registration application fees, product testing, in-country representation, submission preparation consulting and translation of registration documents but not IFU. Costs assume you already have approval for your device in the United States, Europe, Canada or Japan. Does not include cost of implementing or auditing a quality management system compliant with ISO 13485 or US FDA 21 CFR Part 820, if applicable. EmergoGroup.com/India Device classification in India Non-notified IVD Notified Device or IVD How long you should expect to wait after submission until approval is granted.1 < 1 month 9-12 months* Validity period for device registrations.2 3 years** 3 years Renewal should be started this far in advance.3 3 months** 1 year Complexity of the registration process.4 Overall cost of gaining regulatory approval.5 5119-0916 © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 09/2016. Time, Cost, and Complexity of Registration Simple ComplexSimple Complex Low HighLow High * If required, a Technical Presentation or Subject Expert Committee (SEC) Meeting will increase the approval timeline by three to six months. ** Non-notified IVDs do not require registration but do require an import license. This license is valid for three years. This is a simplified overview of the process. The CDSCO may choose to audit your submission and request more documents, which will add time to your approval.
