Sumary sedem formulacion ganador rafc
•
0 gefällt mir•731 views
A continuación os anexo un summary del trabajo que nos permitio obtener un Accesit en el Concurso Cientifico Dr. Esteve 2009 de la Real Academia de Farmacia de Cat - España Autores: Johnny Aguilar, Encarna G, Pilar P. de la Universidad de Barcelona Departamento de Tecnología Farmacéutica
Melden
Teilen
Melden
Teilen
Downloaden Sie, um offline zu lesen
Empfohlen
Empfohlen
A brief discussion on the titleGeneric Drugs; Comparative Dissolution Profile & Discriminative Method for Di...

Generic Drugs; Comparative Dissolution Profile & Discriminative Method for Di...Obaid Ali / Roohi B. Obaid
Weitere ähnliche Inhalte
Was ist angesagt?
A brief discussion on the titleGeneric Drugs; Comparative Dissolution Profile & Discriminative Method for Di...

Generic Drugs; Comparative Dissolution Profile & Discriminative Method for Di...Obaid Ali / Roohi B. Obaid
Was ist angesagt? (19)
Generic Drugs; Comparative Dissolution Profile & Discriminative Method for Di...

Generic Drugs; Comparative Dissolution Profile & Discriminative Method for Di...
The Importance of Amorphous Stability: Mesoporous Silica for Poor Glass Formers

The Importance of Amorphous Stability: Mesoporous Silica for Poor Glass Formers
SEMINAR ON MANUFACTURING AND EVALUATION OF CAPSULES…

SEMINAR ON MANUFACTURING AND EVALUATION OF CAPSULES…
Impact of formulation and process variables on solid-state stability of theop...

Impact of formulation and process variables on solid-state stability of theop...
Impact of Nanosizing on Solubility and Dissolution Rate of Poorly soluble pha...

Impact of Nanosizing on Solubility and Dissolution Rate of Poorly soluble pha...
Ähnlich wie Sumary sedem formulacion ganador rafc
Ähnlich wie Sumary sedem formulacion ganador rafc (20)
Sarno Simone, Polichem an Almirall Company - Minitab e Design of Experiments ...

Sarno Simone, Polichem an Almirall Company - Minitab e Design of Experiments ...
Analytical Techniques In Determining On Line Blend Uniformity In Powder Techn...

Analytical Techniques In Determining On Line Blend Uniformity In Powder Techn...
Stability Indicating HPLC Method Development A Review

Stability Indicating HPLC Method Development A Review
computer aided formulation and development(How to use design expert software)

computer aided formulation and development(How to use design expert software)
Process validation fof Pharmaceutical dosage forms (formulation)

Process validation fof Pharmaceutical dosage forms (formulation)
Process Automation in Pharmaceutical Industry.pptx

Process Automation in Pharmaceutical Industry.pptx
Process validation of tablets, capsules and parentrals

Process validation of tablets, capsules and parentrals
Mehr von Johnny Aguilar Diaz, Ph.D.
Mehr von Johnny Aguilar Diaz, Ph.D. (20)
Kürzlich hochgeladen
Kürzlich hochgeladen (20)
Advantages of Hiring UIUX Design Service Providers for Your Business

Advantages of Hiring UIUX Design Service Providers for Your Business
TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery

TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery
Boost PC performance: How more available memory can improve productivity

Boost PC performance: How more available memory can improve productivity
Tech Trends Report 2024 Future Today Institute.pdf

Tech Trends Report 2024 Future Today Institute.pdf
Strategies for Unlocking Knowledge Management in Microsoft 365 in the Copilot...

Strategies for Unlocking Knowledge Management in Microsoft 365 in the Copilot...
Exploring the Future Potential of AI-Enabled Smartphone Processors

Exploring the Future Potential of AI-Enabled Smartphone Processors
TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments

TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments
Automating Google Workspace (GWS) & more with Apps Script

Automating Google Workspace (GWS) & more with Apps Script
Boost Fertility New Invention Ups Success Rates.pdf

Boost Fertility New Invention Ups Success Rates.pdf
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
Understanding Discord NSFW Servers A Guide for Responsible Users.pdf

Understanding Discord NSFW Servers A Guide for Responsible Users.pdf
Axa Assurance Maroc - Insurer Innovation Award 2024

Axa Assurance Maroc - Insurer Innovation Award 2024
What Are The Drone Anti-jamming Systems Technology?

What Are The Drone Anti-jamming Systems Technology?
Sumary sedem formulacion ganador rafc
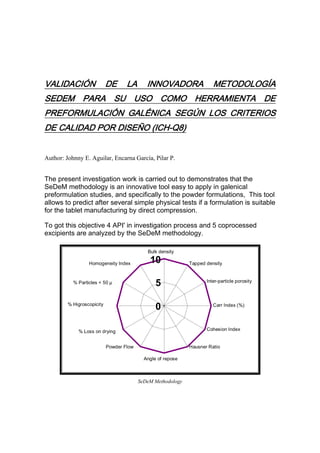
- 1. VALIDACIÓN DE LA INNOVADORA METODOLOGÍA SEDEM PARA SU USO COMO HERRAMIENTA DE PREFORMULACIÓN GALÉNICA SEGÚN LOS CRITERIOS DE CALIDAD POR DISEÑO (ICH-Q8) Author: Johnny E. Aguilar, Encarna García, Pilar P. The present investigation work is carried out to demonstrates that the SeDeM methodology is an innovative tool easy to apply in galenical preformulation studies, and specifically to the powder formulations, This tool allows to predict after several simple physical tests if a formulation is suitable for the tablet manufacturing by direct compression. To got this objective 4 API' in investigation process and 5 coprocessed excipients are analyzed by the SeDeM methodology. Bulk density Homogeneity Index 10 Tapped density % Particles < 50 µ 5 Inter-particle porosity % Higroscopicity 0 Carr Index (%) % Loss on drying Cohesion Index Powder Flow Hausner Ratio Angle of repose SeDeM Methodology
- 2. After the analysis of these products a profile is obtained for each of them which helps to determine the characteristics of the product, which previously they have been stipulated in 5 parameters: dimensions, compressibility, lubrication/stability and lubrication/dosage. When the powder formulation are characterized, it could be distinguished those parameters that must be improved if tablet by direct compression is planned. In addition this characterization can constitute a library in the laboratory of pharmaceutical technology, consequently in next the development it can be consulted without having to repeat the tests. It is just necessary to carry out for new products.
- 3. This design under control of the formulations, facilitates that the SeDeM methodology is being positioned as a fundamental and indispensable tool at the time of development of tablet by direct compression under the philosophy of quality by design (ICH Q8: Quality by Design) since each of their parameters forms entrance variables are analyzed, when the development of the design of the space is carried out.
- 4. Respect to the API' s in investigational process and the is conclude that 2 API' s and 2 coprocessed excipients have a good index of compressibility, which makes them suitable to be used in the manufacturing of tablets by direct compression.
