Ni in h2 so4 graphic organizer key
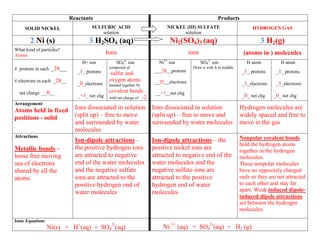
- 1. Reactants Products SOLID NICKEL SULFURIC ACID NICKEL (III) SULFATE HYDROGEN GAS solution solution 2 Ni (s) 3 H2SO4 (aq) Ni2(SO4)3 (aq) 3 H2(g) What kind of particles? Atoms Ions ions (atoms in ) molecules H+ ion SO42- ion Ni3+ ion SO42- ion H atom H atom # protons in each _28___ composed of Draw it with S in middle. _1_ protons ___28__protons _1_ protons _1_ protons sulfur and # electrons in each _28__ oxygen atoms __25__electrons _0_electrons bonded together by _1_electrons _1_electrons net charge __0__ covalent bonds __+3__net chg _+1_ net chg with net charge of -2 _0_ net chg _0_ net chg Arrangement Atoms held in fixed Ions dissociated in solution Ions dissociated in solution Hydrogen molecules are positions - solid (split up) – free to move (split up) – free to move and widely spaced and free to and surrounded by water surrounded by water molecules move in the gas molecules Attractions Nonpolar covalent bonds Ion-dipole attractions – Ion-dipole attractions – the hold the hydrogen atoms Metallic bonds – the positive hydrogen ions positive nickel ions are together in the hydrogen loose free moving are attracted to negative attracted to negative end of the molecules. sea of electrons end of the water molecules water molecules and the These nonpolar molecules shared by all the and the negative sulfate negative sulfate ions are have no oppositely charged atoms ions are attracted to the attracted to the positive ends so they are not attracted positive hydrogen end of hydrogen end of water to each other and stay far water molecules molecules apart. Weak induced dipole- induced dipole attractions act between the hydrogen molecules. Ionic Equations Ni(s) + H+(aq) + SO42-(aq) Ni 3+ (aq) + SO42-(aq) + H2 (g)
- 2. Ni(s) + H+(aq) Ni 3+ (aq) + H2 (g) Help for the ionic equations From the molecular equation to the ionic equation 2 Ni (s) 3 H2SO4 (aq) Ni2(SO4)3 (aq) 3 H2(g) The only change is splitting up the ions that are in solution. So if it is not in solution (aq) – leave it the same and if it is in solution and IONS - split them up. Do NOT include the subscripts (little numbers) unless they are part of the polyatomic ion. Example – sulfate ion is four oxygen atoms and one sulfur atom so you must include the four SO42- Do show the charge on the ion.
- 3. Lab 5 Worksheet Name _________________________________ period ___ A nickel is added to sulfuric acid. A single replacement reaction occurs. Nickel (III) sulfate forms. Bubbles form and rise to the surface. When the bubbles stop forming, the nickel is much smaller than before. 1. Complete the analysis on the back before proceeding. Use the ionic equations Ni(s) + H+(aq) + SO42-(aq) Ni 3+ (aq) + SO42-(aq) + H2 (g) 2. What happened to the nickel. Write a half-reaction. Write down the nickel before and after from the ionic equation. Ask yourself – atom, ion, or molecule Ask yourself – how many protons? How many electrons? Then look – what changed? Ni(s) Ni 3+ (aq) + 3e- atom ion 28 p 28 p 28e- 25 e- lost three electrons -- OXIDIZED 0 +3 3. What happened to the hydrogen. Write a half-reaction. Write down the hydrogen before and after from the ionic equation. Ask yourself – atom, ion, or molecule Ask yourself – how many protons? How many electrons? Then look – what changed? H+(aq) H2 (g) ion molecule (atoms in) 1p 1p 1p (two H atoms) 0 e- 1 e- 1 e- (sharing a pair of electrons) +1 ` 0 0 Gained electrons --- REDUCED 4. What happened to the sulfate? NOTHING - it was an ion in solution attracted to water molecules and it still is an ion in solutions attracted water molecules. 5. Based upon the description of the reaction, why did the reaction stop? How do you know? Since there is still nickel left after the bubbling stops, we must have leftover nickel. We must have run out of sulfuric acid, so that is why the reaction stopped. Sulfuric acid is the LIMITING REACTANT – it limits how much hydrogen gas you can make. 6. Is the remaining solution still acidic? Explain. No, we ran out of acid.
- 4. The mass of nickel was 5.061 grams and 25 mL of 2.0 M H2SO4 solution was used. 7. How many moles of nickel are present initially?. Ask yourself – what are we looking for? Put it at the right. Ask yourself – what is the starting info closest to what I am looking for? Write it at the left. Then look – what factors of one (top equals bottom) can I use to turn the starting point into the ending point? 5.0161 g Ni 1 mole Ni Moles Ni 58.71 g Ni What are we looking for? Put it at the right. what is the starting info closest to what I am looking for? 8. How many moles of sulfuric acid are present initially? 9. If all the nickel reacts, how many moles of hydrogen gas can be produced? 10. If all the sulfuric acid reacts, how many moles of hydrogen gas can be produced? After the reaction, the remaining nickel was removed from solution. The nickel was rinsed over the beaker and the solution was decanted. The solution was heated to dryness. The mass of nickel removed from the solution was 3.042 grams. The mass of the dried nickel sulfate solid was 13.084 g. 12. Calculate the mass of nickel that actually reacted. 13. Use the mass of nickel that actually reacted to calculate the mass of nickel (III) sulfate that should have been produced. 14. Why was the leftover nickel rinsed over the beaker. 15. Why was the mass of the remaining nickel measured? 16. Why was the remaining solution dried?
- 5. ANALYSIS OF THE REACTION OF ALUMINUM IN HYDROCHLORIC ACID THE CHANGES ALUMINUM ALUMINUM before ALUMINUM after Notes- What kind of particle? What kind of particle? What happened to the aluminum? How many protons? How many protons? How many electrons? How many electrons? Chemistry term for this change net charge? net charge? half reaction CHLORINE CHLORINE before CHLORINE after Notes- What kind of particle? What kind of particle? What happened to the aluminum? How many protons? How many protons? How many electrons? How many electrons? Chemistry term for this change net charge? net charge? HYDROGEN IONS HYDROGEN before HYDROGEN after Notes- What kind of particle? What kind of particle? What happened to the hydrogen? How many protons? How many protons? How many electrons? How many electrons? Chemistry term for this change net charge? net charge? half reaction SINGLE REPLACEMENT REACTIONS.