Units Of Concenration
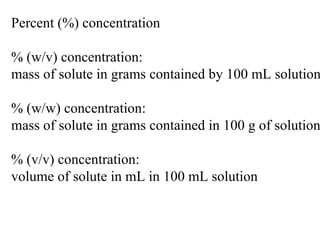
- 1. Percent (%) concentration % (w/v) concentration: mass of solute in grams contained by 100 mL solution % (w/w) concentration: mass of solute in grams contained in 100 g of solution % (v/v) concentration: volume of solute in mL in 100 mL solution
- 2. parts per million (ppm) are used to express the concentrations of very dilute solution A solution whose solute concentration is 1 ppm contains 1 g of solute for each million (10 6 ) grams of solution or, equivalently, 1 mg of solute per kilogram of solution A 2.5-g sample of groundwater was found to contain 5.4 g of Zn 2+ . What is the concentration of Zn 2+ in parts per million?
- 3. Mole Fraction, Molarity, and Molality are concentration expressions based on the number of moles of one or more components of the solution The symbol X is used for mole fraction , with a subscript to indicate the component of interest. The mole fraction of HCl is represented as X H Cl . The sum of the mole fractions of all components of a solution must equal 1 A solution of hydrochloric acid contains 36 percent HCl by mass. Calculate the mole fraction of HCl in the solution.
- 4. Molarity of a solute in a solution is defined as the number of moles of solute per liters of solution A 2.00L solution of hydrochloric acid contains 36 g of HCl. Calculate the molarity of HCl in the solution. M HCl = ---------------- 0.99 mol 2.00 L = 0.49 5 = 0.50 M
- 5. The molality of a solution, denoted m, is defined as the number of moles of solute per kilogram of solvent A solution of hydrochloric acid contains 36 percent HCl by mass. Calculate the molality of HCl in the solution. (c) What additional information would you need to calculate the molarity of the solution? Mass of water = 100 gSolu – 36 g HCl = 64 g
- 6. (a) Calculate the mole fraction of NaOCl in a commercial bleach solution containing 3.62 mass percent NaOCl in water. = 0.00900
- 7. (b) What is the molality of a solution made by dissolving 36.5 g of naphthalene, C 10 H 8 , in 420 g of toluene, C 7 H 8 ? (b) 0.678 m = 0.28477 / .420 0.678 024
- 8. Given that the density of a solution of 5.0 g of toluene and 225 g of benzene is 0.876 g/mL, calculate (a) the molarity of the solution; (b) the mass percentage of solute.
- 9. Calculate the molality of a solution that contains 25 g of H 2 SO 4 dissolved in 80. g of H 2 O
- 10. Calculate the molality of a 10.0% H 3 PO 4 solution in water
- 11. Calculate the molality of a solution that contains 51.2 g of naphthalene, C 10 H — , in 500. mL of carbon tetrachloride. The density of CCl 4 is 1.60 g/mL
- 12. If 8.32 grams of methanol, CH 3 OH, are dissolved in 10.3 grams of water, what is the mole fraction of methanol in the solution?
- 13. What is the molarity of 2500. mL of a solution that contains 160. grams of NH 4 NO 3 ?
- 14. 11-1Acid and base Reactions 100. mL of 0.100 M HCl solution and 100. mL of 0.100 M NaOH are mixed. What is the molarity of the salt in the resulting solution? Assume that the volumes are additives. 1. Balance Chemical Equation HCl + NaOH H 2 O + NaCl Reaction Ratio: 1 mol 1 mol 1 mol 1 mol Start: Change: For HCl: (100 mL)(0.100mol/L) = 10.0 m mol HCl 10.0 m mol For NaOH = (100. mL)(0.100mol/L) = 10.0 mmol 10.0 m mol 0 For NaCl: moles of NaCl at the end of reaction = moles of HCl at beginning = 10.0 m mol -10.0 -10.0 After reaction: 0.00 0.00 10.0 M NaCl = ---------------------------- 10.0 m mol NaCl (100+100) m L solution = 0.0500 10.0
- 15. 11-1Acid and base Reactions 80. mL of 0.100 M HCl solution and 100. mL of 0.100 M NaOH are mixed. What is the molarity of the salt in the resulting solution? Assume that the volumes are additives. 1. Balance Chemical Equation HCl + NaOH H 2 O + NaCl Reaction Ratio: 1 mol 1 mol 1 mol 1 mol Start: Change: For HCl: (80 mL)(0.100mol/L) = 8.0 m mol HCl 8.0 m mol For NaOH = (100. mL)(0.100mol/L) = 10.0 mmol 10.0 m mol 0 For NaCl: moles of NaCl at the end of reaction = moles of HCl at beginning = 8.0 m mol -8.0 -8.0 After reaction: 0.00 2.0 8.0 M NaCl = ---------------------------- 8.0 m mol NaCl (100+80) m L sol = 0.044 M NaOH = ------------------------ 2.0 mmol NaOH 180 mL solution = 0.011 8.0 m mol
- 16. 100. mL of 0.100 M HCl solution and 100. mL of 0.80 M NaOH are mixed. What is the molarity of the salt in the resulting solution? Assume that the volumes are additives. 1. Balance Chemical Equation HCl + NaOH H 2 O + NaCl Reaction Ratio: 1 mol 1 mol 1 mol 1 mol Start: Change: For HCl: (100 mL)(0.100mol/L) = 10.0 m mol HCl 10.0 m mol For NaOH = (100. mL)(0.80mol/L) = 8.0 mmol 8.0 m mol 0 For NaCl: moles of NaCl at the end of reaction = moles of NaOH at beginning = 8.0 m mol -8.0 -8.0 After reaction: 2.0 0.0 8.0 +8.0 M NaCl = ---------------------------- 8.0 m mol NaCl (100+100) m L solution = 0.040 M HCl = ---------------------------- 2.0 m mol NaCl (100+100) m L solution = 0.010
- 17. 100. mL of 0.100 M HCl solution and 100. mL of 0.80 M NaOH are mixed. What is the molarity of the salt in the resulting solution? Assume that the volumes are additives. 1. Balance Chemical Equation HCl + NaOH H 2 O + NaCl Reaction Ratio: 1 mol 1 mol 1 mol 1 mol Start: Change: For HCl: (100 mL)(0.100mol/L) = 10.0 m mol HCl 10.0 m mol For NaOH = (100. mL)(0.80mol/L) = 80. mmol 80. m mol 0 For NaCl: moles of NaCl at the end of reaction = moles of NaOH at beginning = 10. m mol -10.0 -10.0 After reaction: 0.00 70. 8.0 +10.0 M NaCl = ---------------------------- 10. m mol NaCl (100+100) m L solution = 0.050 M NaOH = ---------------------------- 70. m mol NaCl (100+100) m L solution = 0.035
- 18. 100. mL of 1.00 M H 2 SO 4 solution is mixed with 200. mL of 1.00 M KOH. What is the molarity of the salt in the resulting solution? Assume that the volumes are additives. 1. Balance Chemical Equation H 2 SO 4 + 2KOH 2H 2 O + K 2 SO 4 Reaction Ratio: 1 mol 2 mol 2 mol 1 mol Start: Change: Moles of H 2 SO 4 : (100 mL)(1.00mol/L) = 100 m mol 100 m mol mols KOH = (200. mL)(1.00mol/L) = 200 mmol 200 m mol 0 moles of K 2 SO 4 at the end of reaction = moles of H 2 SO 4 at beginning = 100 m mol -200 -100 After reaction: 0.00 0.0 100 M H 2 SO 4 = --------------------- 100 m mol K 2 SO 4 (100+200) m L sol = 0.333 100 m mol
- 19. Titration Titration is a process in which a solution of one reactant, titrant, is carfully added