Ch. 5 lecture
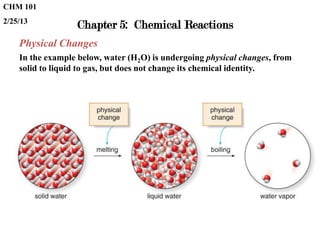
- 1. CHM 101 2/25/13 Chapter 5: Chemical Reactions Physical Changes In the example below, water (H2O) is undergoing physical changes, from solid to liquid to gas, but does not change its chemical identity.
- 2. Chemical Reactions Chemical changes occur when one substance is converted into a different substance, thus changing the chemical identity. H2 (g) + O2 (g) 2H2O (g) In a chemical reaction, old bonds are broken and new bonds are formed.
- 3. Chemical Reactions A chemical equation gives the chemical formulas of the reactants on the left of the arrow and the products on the right. O2 (g) Reactant(s) Product(s) C(s) CO2 (g) Symbols used in chemical equations show the states of the reactants and products and the reaction conditions.
- 4. Chemical Reactions In a balanced chemical reaction, atoms are not gained or lost. The number of reactant atoms is equal to the number of product atoms. This is supported by the Law of Conservation of Mass.
- 5. Chemical Reactions In a balanced chemical equation, numbers called coefficients are used in front of one or more formulas. Al + S Al2S3 Not Balanced 2Al + 3S Al2S3 Balanced 2 Al = 2 Al 3S = 3S
- 6. Chemical Reactions To balance the following equation, Fe3O4(s) + H2(g) Fe(s) + H2O(l) • work on one element at a time. • use only coefficients in front of formulas. • do not change any subscripts. Fe: Fe3O4(s) + H2(g) 3Fe(s) + H2O(l) O: Fe3O4(s) + H2(g) 3Fe(s) + 4H2O(l) H: Fe3O4(s) + 4H2(g) 3Fe(s) + 4H2O(l)
- 7. Chemical Reactions 1. Write the equation with the correct formulas. NH3(g) + O2(g) NO(g) + H2O(g) 2. Determine if the equation is balanced. 3. Balance with coefficients in front of formulas. 4. Check that atoms of each element are equal in reactants and products.
- 8. Equations with Polyatomic Ions Chemical Reactions
- 9. Chemical Reactions MgCl2(aq) + Na3PO4(aq) NaCl(aq) + Mg3(PO4)2(s) Balance PO43- as a unit MgCl2(aq) + 2Na3PO4(aq) NaCl(aq) + Mg3(PO4)2(s) Balance Mg and Cl 3MgCl2(aq) + 2Na3PO4(aq) 6NaCl(aq) + Mg3(PO4)2(s) Other hints: 1. Save O and H until the end. Balance other atoms first. 2. Save atoms that are “alone” (H2, Cl2, Na, etc) until the end. You can always adjust them without upsetting the balance of other atoms.
- 10. Chemical Reactions 1. Write the equation with the correct formulas. C6H14 (l) + O2 (g) CO2 (g) + H2O (l) 2. Determine if the equation is balanced. 3. Balance with coefficients in front of formulas. 4. Check that atoms of each element are equal in reactants and products.
- 11. Chemical Reactions What Can We Learn From a Balanced Reaction? 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) 4 molecules of NH3 react with 5 molecules of O2 to produce 4 molecules of NO and 6 molecules of H2O. We rarely think about reactions in terms of a few molecules. Instead, we talk about… 400,000,000,000 trillion molecules!!! We need a way to talk about this HUGE number….
- 12. Chemical Reactions A collection term states a specific number of items. • 1 dozen donuts = 12 donuts • 1 ream of paper = 500 sheets • 1 case = 24 cans None of these are large enough to help with our 400,000,000,000 trillion molecules….
- 13. The MOLE
- 14. Moles & Chemical Reactions A mole is a collection that contains 6.02 x 1023 “things”. The “things” could be atoms, ions, molecules, etc. It’s the same number of particles as there are carbon atoms in 12.0 g of carbon-12. 6.02 x 1023 is called “Avogadro’s number” 1 mole element Number of Atoms 1 mole C = 6.02 x 1023 C atoms 1 mole Na = 6.02 x 1023 Na atoms 1 mole Au = 6.02 x 1023 Au atoms
- 15. Moles & Chemical Reactions A mole of a covalent compound has Avogadro’s number of molecules. 1 mole CO2 = 6.02 x 1023 CO2 molecules 1 mole H2O = 6.02 x 1023 H2O molecules A mole of an ionic compound contains Avogadro’s number of formula units. 1 mole NaCl = 6.02 x 1023 NaCl formula units 1 mole K2SO4 = 6.02 x 1023 K2SO4 formula units
- 16. Moles & Chemical Reactions Avogadro’s number 6.02 x 1023 can be written as an equality and two conversion factors. Equality: 1 mole = 6.02 x 1023 particles Conversion Factors: 6.02 x 1023 particles or 1 mole 1 mole 6.02 x 1023 particles
- 17. Moles & Chemical Reactions Avogadro’s number is used to convert moles of a substance to particles. How many Cu atoms are in 0.50 mole Cu? 0.50 mole Cu x 6.02 x 1023 Cu atoms = 3.0 x 1023 Cu atoms 1 mole Cu Avogadro’s number is used to convert particles of a substance to moles. How many moles of CO2 are in 2.50 x 1024 molecules CO2? 2.50 x 1024 molecules CO2 x 1 mole CO2 = 4.15 mol CO2 6.02 x 1023 molecules CO2
- 18. Moles & Chemical Reactions If we have 1 dozen H2O molecules, then… We have 2 dozen H atoms (24) and 1 dozen O atoms (12). H2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O H 2 O If we have 1 mol of H2O molecules, then… We have 2 mol H atoms and 1 mol O atoms.
- 19. Moles & Chemical Reactions OH H2C H C O H Glucose C OH H C H C6H12O6 HO C C OH H OH In 1 molecule: 6 atoms C 12 atoms H 6 atoms O In 1 mole of molecules: 6 mole C 12 mole H 6 mole O
- 20. Moles & Chemical Reactions The subscripts are used to write conversion factors for moles of each element in 1 mole compound. For aspirin, C9H8O4, the following factors can be written: 9 mol C 8 mol H 4 mol O 1 mol C9H8O4 1 mol C9H8O4 1 mol C9H8O4 or 1 mol C9H8O4 1 mol C9H8O4 1 mol C9H8O4 9 mol C 8 mol H 4 mol O
- 21. Moles & Chemical Reactions A. How many moles of O atoms are in 0.150 mol aspirin C9H8O4? ANS: 0.600 mol B. How many H atoms are in 0.150 mol aspirin C9H8O4? ANS: 7.23 x 1023 atoms H
- 22. The Mole & Mass 1 dozen eggs: 12 eggs 1 dozen bags of sugar: 12 bags of sugar Do you expect these to have the same mass? No, because the mass per egg is different than the mass per bag of sugar. 1 mole S atoms: 6.022 x 1023 S atoms 1 mole Fe atoms: 6.022 x 1023 Fe atoms Do you expect these to have the same mass? No, because the mass per S atom is different than the mass per Fe atom.
- 23. Some One-mole Quantities 32.1 g 55.9 g 58.5 g 294.2 g 342.0 g Each sample is 1 mole of the substance, but each has a very different mass.
- 24. Molar Mass The molar mass is the mass of one mole of an element or compound. It is the atomic mass expressed in grams.
- 25. Molar Mass Molar mass conversion factors relate grams and moles of an element or compound. Example: Write molar mass factors for sodium, Na. Molar mass: 1 mol Na = 22.99 g Conversion factors: 22.99 g Na and 1 mole Na 1 mole Na 22.99 g Na
- 26. Molar Mass Molar mass factors are used to convert between the grams of a substance and the number of moles. Grams Molar mass factor Moles Aluminum is often used to build lightweight bicycle frames. How many grams of Al are in 3.00 mole Al? Molar mass equality: 1 mole Al = 27.0 g Al Setup with molar mass as a factor: 3.00 mole Al x 27.0 g Al = 81.0 g Al 1 mole Al molar mass factor for Al
- 27. Molar Mass The molar mass of a compound is the sum of the molar masses of the elements in the formula. Example: Calculate the molar mass of CaCl2. Element Number of Atomic Mass Molar Mass Atoms Ca 1 40.1 g/mole 40.1 g/mol Cl 2 35.5 g/mole 71.0 g/mol CaCl2 111.1 g/mole
- 28. Molar Mass Calculate the molar mass of K3PO4. Element Number of Atoms Atomic Mass Molar Mass K 3 39.1 g/mole 117.3 g/mol P 1 31.0 g/mole 31.0 g/mol O 4 16.0 g/mole 68.0 g/mol K3PO4 212.3 g/mole
- 29. Molar Mass Allyl sulfide C6H10S is a compound that has the odor of garlic. How many moles of C6H10S are in 225 g? ANS: 1.97 moles C6H10S
- 30. Molar Mass A molar mass factor and Avogadro’s number convert grams to particles. molar mass Avogadro’s number g mole particles • OR particles to grams. Avogadro’s molar mass number particles mole g If the odor of C6H10S can be detected from 2 x 10-13 g in one liter of air, how many molecules of C6H10S are present? ANS: 1 x 109 molecules C6H10S
- 31. Law of Conservation of Mass The Law of Conservation of Mass indicates that in an ordinary chemical reaction, matter cannot be created nor destroyed. • No change in total mass occurs in a reaction. • Mass of products is equal to mass of reactants. 2Ag (s) + S (s) Ag2S (s) 2 moles Ag + 1 moles S = 1 mole Ag2S 2 (107.9 g) + 1(32.1 g) = 1 (247.9 g) 247.9 g reactants = 247.9 g product
- 32. Chemical Reactions Information from balanced chemical equations – The balanced equation tells us the relative numbers of molecules or moles that react and that are produced. Consider the following equation: 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) This equation can be read in “atoms/molecules/units” by placing the word “atoms/molecules/units” between each coefficient and formula. 4 atoms Fe + 3 molecules O2 2 units Fe2O3 This equation can also be read in “moles” by placing the word “moles” between each coefficient and formula. 4 moles Fe + 3 moles O2 2 moles Fe2O3
- 33. Chemical Reactions Stoichiometry – the study of the mass-mole-number relationship of chemical formulas and reactions. Mole Ratios – Use stoichiometric coefficients in a balanced chemical equation to relate the number of moles of reactants and products to each other. C3H8 (g) + 5O2 (g) 3CO2 (g) + 4H2O (g) Possible Mole Ratios: 1 mole C3 H 8 1 mole C3H 8 3 moles CO 2 5 moles O 2 3 moles CO 2 5 moles O 2 **Use coefficients to relate any 2 reactants, a reactant to a product, or any 2 products. But it only works for moles (or atoms/molecules), not mass!
- 34. Chemical Reactions Using Mole Ratios Using the balanced reaction below for the combustion of propane, calculate the number of moles of CO2 produced if 0.320 mol C3H8 are burned in excess O2. C3H8 (g) + 5O2 (g) 3CO2 (g) + 4H2O (g) 0.320 mol ? moles mole ratio moles C3H8 moles CO2 1 mole C3H 8 mole ratio: 3 moles CO 2 3 mol CO 2 0.320 mol C3H 8 × = 0.960 mol CO 2 1 mole C3H 8
- 35. Chemical Reactions Using Mole Ratios Using the balanced reaction below for the combustion of propane, calculate the number of moles of CO2 produced if 3.52 g C3H8 are burned in excess O2. C3H8 (g) + 5O2 (g) 3CO2 (g) + 4H2O (g) 3.52 g ? moles molar mass mole ratio mass C3H8 moles C3H8 moles CO2 molar mass C3H8 1 mole C3H 8 C: 12.01 g/mol * 3 = 36.03 g/mol 3.52 g C3H 8 × = 0.0798 mol C3 H 8 44.11 g H: 1.01 g/mol * 8 = 8.08 g/mol mole ratio: 1 mole C3 H 8 44.11 g/mol 3 moles CO 2 3 mol CO 2 0.0798 mol C3 H 8 × = 0.239 mol CO 2 1 mole C3 H 8
- 36. Chemical Reactions Using Mole Ratios Using the unbalanced reaction below (BALANCE FIRST), calculate the mass of SO2 (g) formed when 10.0 moles of Cu2S react with excess O2. Cu2S (s) + O2 (g) Cu2O (s) + SO2 (g) 10.0 mol ? mass mole ratio molar mass mol Cu2S moles SO2 mass SO2 ANS: 641 g SO2 Using the same reaction, calculate the mass of O2 (g) required to completely react with 10.0 moles of Cu2S. mole ratio molar mass mol Cu2S moles O2 mass O2 ANS: 4.80 x 102 g O2
- 37. Chemical Reactions Percent Yield A balanced equation allows us to predict the amount of products that should form. But we do not always get the predicted amount. WHY? Theoretical Yield – predicted amount of product based on stoichiometric coefficients vs. Actual Yield – quantity of product actually obtained from the reaction. Percent Yield – measures the efficiency of a reaction, ratio of actual to theoretical yield. actual yield Percent Yield = ×100 theoretical yield
- 38. Chemical Reactions Percent Yield Marble (CaCO3) reacts with hydrochloric acid (HCl) to form calcium chloride, water, and carbon dioxide. a. If 10.0 g of marble reacts, what mass of CO2 should form? b. If 3.65 g of CO2 actually forms, what is the percent yield for the reaction? CaCO3 (s) + 2HCl (aq) CaCl2 (aq) + H2O (l) + CO2 (g) 10.0 g theoretical mass: ? actual mass: 3.65 g molar mass mole ratio molar mass mass CaCO3 mol CaCO3 mol CO2 mass CO2 theoretical mol 1 mol CO 2 a) 10.0 g CaCO 3 × = 0.09991 mol CaCO 3 × = 0.09991 mol CO 2 mass 100.09 g 1 mol CaCO 3 44.01g 0.09991mol CO 2 × = 4.397 g CO 2 mol b) actual yield 3.65 g Percent Yield = ×100 Percent Yield = ×100 = 83 .0% theoretical yield 4.397 g
- 39. Chemical Reactions Oxidation-Reduction Reactions What is this? 4Fe (s) + 3O2 (g) 2Fe2O3 (s) electrons Fe O Fe3+ O2- + + atom atom atom atom This is an example of an oxidation-reduction reaction: a reaction in which there is a net movement of electrons from one reactant to another.
- 40. Chemical Reactions Oxidation-Reduction Reactions Loses e- Mn (s) + Fe3+ Fe (s) + Mn2+ (aq) gains e- Oxidation: the loss of electrons. Reduction: the gain of electrons. An atom becomes more positive. An atom becomes less positive. Mn was oxidized to Mn2+. Fe3+ was reduced to Fe. In every oxidation-reduction reaction a reactant is oxidized and a reactant is reduced. Oxidizing Agent: the reactant Reducing Agent: the reactant causing causing the oxidation (it is reduced). the reduction (it is oxidized) Fe3+ is the oxidizing agent. Mn is the reducing agent.
- 41. Chemical Reactions Oxidation-Reduction Reactions For the reaction below, identify the substance that was oxidized. Identify the substance that was reduced. Identify the oxidizing agent and the reducing agent. 2H+ (aq) + Zn (s) H2 (g) + Zn2+ (aq) Write half-reactions for the oxidation and reduction steps. reduction 2H+ + 2e H2 (g) oxidation Zn (s) Zn2+ (aq) + 2e The number of electrons lost (oxidation) must be equal to the number of electrons gained (reduction).
- 42. Chemical Reactions Oxidation and Reduction in Biological Systems CH3OH H2CO + 2H+ Oxidation (loss of H) 2H2CO + O2 2H2CO2 Oxidation (gain of O)
