2
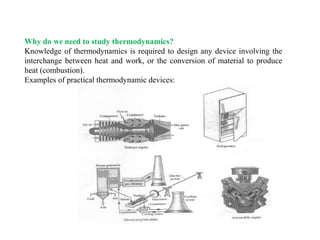
- 1. Why do we need to study thermodynamics? Knowledge of thermodynamics is required to design any device involving the interchange between heat and work, or the conversion of material to produce heat (combustion). Examples of practical thermodynamic devices:
- 2. What is thermodynamics? • The study of the relationship between work, heat, and energy. • Deals with the conversion of energy from one form to another. • Deals with the interaction of a system and it surroundings. System - identifies the subject of the analysis by defining a boundary Surroundings - everything outside the system boundary. As an example, consider an Internal Combustion (IC) Engine
- 3. Closed System - fixed non-changing mass of fluid within the system, i.e., no mass transfer across the system boundary but can have energy exchange with the surroundings. Example: piston-cylinder assembly Isolated System - a system that does not interact at all with the surroundings, e.g., no heat transfer across system boundary Open System (Control Volume) - fixed volume in space, mass and energy exchange permitted across the system boundary. Example: jet engine System Properties – macroscopic characteristics of a system to which a numerical value can be assigned at a given time without knowledge of the history of the system, e.g., mass, volume, pressure There are two types of properties: Extensive – the property value for the system is the sum of the values of the parts into which the system is divided (depends on the system size) e.g., mass, volume, energy Intensive – the property is independent of system size (value may vary throughout the system), e.g., pressure, temperature
- 4. Units – SI used exclusively Fundamental units: Mass kilograms kg Length meter m Time seconds s T temperature Celsius/Kelvin oC/oK Derived units: Force (F) Newton N Pressure (P) Pascal Pa Energy (E) Joule J Newton’s Law states: Force = mass x acceleration F = m ⋅ a [N] = [kg] [m/s2] 1 N = 1 kg⋅m/s2 P = F / A [Pa] [kg⋅m/s2] [m2] 1 Pa = 1 kg/m⋅s2 E = F ⋅ x [J] = [kg⋅m/s2] [m] 1 J = 1 kg⋅m2/s2
- 5. Property Definitions In order to speak of an intrinsic property “at a point” we must treat matter as a continuum, i.e., matter is distributed continuously in space • In classical thermodynamics a point represents the smallest volume V’ for which matter can be considered a continuum. • The value of the property represents an average over this volume V’. At any instant the density, ρ, at a point is defined as ρ lim → = V V
- 6. Properties of a Pure, Simple Substance A pure substance is one of uniform and invariable chemical composition For our purposes a simple substance is taken as one for which if the values of two intensive properties are known all the other properties can be found, e.g., u= f(T,P) P-v-T Relationship A material can exist in the 1. solid phase 2. liquid phase 3. vapor (gas) phase 4. a mixture of the phases at equilibrium, e.g., melting, vaporization or sublimation Through experiments it is known that temperature and specific volume can be considered as independent and pressure determined as a function of these two: P= p(T,v) The graph of such a function is a surface, the P-v-T surface.
- 7. P-v-T surface of a substance that contracts on freezing (common metals) Note Note the step decrease in specific volume (step increase in density) when going from liquid to solid
- 8. P-v-T surface of a substance that expands on freezing (water) Note the step increase in specific volume (step decrease in density) when going from liquid to solid 3-D surface plot not very useful, more instructive to look at 2-D projections
- 9. P-T plot for substance that expands on freezing Only single-phase regions observed Two-phase regions appear as lines (edge view) Triple point represents edge view of the triple line where all three phases co-exist
- 10. T-v plot for substance that expands on freezing (water) Constant pressure lines are known as isobars The critical point defines the maximum temperature, i.e., above the critical temperature a liquid and vapor cannot co-exist at equilibrium
- 12. Second Law of Thermodynamics It is an observed fact that certain processes can only proceed spontaneously in one direction (hot coffee gets colder) The following does not occur Another example, connecting high pressure tank with a low pressure tank: where PE is the final pressure
- 13. The evolution of the Second Law The First Law of Thermodynamics is used to calculate end states of a system as it evolves, it does not answer the following questions: 1) In what direction does a spontaneous process go 2) What is the maximum possible work The Second Law of Thermodynamics starts with a simple principal concerning the direction of heat flow and evolves into developing a new property called entropy (S) Clausius Statement It is impossible for a system to operate in such a way that the sole result is the transfer of heat from a cold to a hot body Kelvin Planck Statement It is impossible for a system that operates in a cycle to generate work while transferring heat with a single reservoir
- 14. Recall, a reservoir is a body that has so much thermal capacity that its temperature doesn’t change when heat transfer occurs To illustrate the equivalence of the two statements consider the following: Connect two thermal reservoirs with high thermal conductivity metal and assume Q1 heat flows from TC to TH which according to Clausius is not possible Then place a heat engine between TH and TC that draws Q1 heat from the TH reservoir and dumps Q2 heat to the TC reservoir Then place a heat engine between TH and TC that draws Q1 heat from the TH reservoir and dumps Q2 heat to the TC reservoir
- 15. This is equivalent to This engine takes heat from one reservoir (Tc) to produce work this is not possible according to K-P statement and thus demonstrating the equivalency of the two statements Heat Engines Work can easily be converted to heat and other forms of energy, but converting heat into work is not so easy Converting heat into work requires a heat engine Earliest heat engine operated on steam: Process I - add steam into the piston-cylinder to raise the pressure above atmospheric pressure and thus push the piston down Process II - add water to condense the steam and lower the pressure below atmospheric pressure so the piston is “pulled” back up
- 16. Thermal Efficiencies Basic characteristics of heat engine are: 1) Receive heat, QH, from a high temperature source 2) Convert part of this heat to work, W 3) Reject the remaining waste heat, QC, to a low temperature sink 4) Operate on a cycle These devices involve a working fluid to and from which heat is transferred First Law applied to the heat engine cycle yields The efficiency of the cycle is defined as
- 17. Maximum possible work corresponds to Thermal efficiency is the ratio of the work done and the heat input, substituting for W For an internal combustion (IC) engine heat is supplied by combustion QH and heat rejected through the exhaust QC, typically the thermal efficiency of combustion is around 30% Other engine mechanical inefficiencies translate into an even lower overall efficiency
- 18. Refrigerators A refrigerator takes heat from a hot reservoir (hot room) and dumps it into a cooler reservoir (cooler outdoors). The refrigeration cycle is the opposite of the heat engine requiring work input Note: this is not inconsistent with Clausius’ statement because the heat transfer from the cold to the hot reservoir is not spontaneous. Applying First Law to the refrigeration cycle Coefficient of performance (COP) β defined as Typical values of β are 3-4
- 19. Reversible and Irreversible Processes Have shown that no engine can have 100% efficiency, the question becomes what is the maximum efficiency a heat engine can achieve? The maximum efficiency will correspond to a cycle consisting of a series of idealized reversible processes A reversible process is one which the system and its surroundings can be returned to their respective original states at the end of the reverse process If the system and surroundings cannot be returned to their respective original states the process is termed irreversible and the process is said to involve irreversibilities. A process is internally reversible if no irreversibilities occur inside the system boundary, irreversibilities may occur outside the system The Carnot Cycle The most efficient cycle is one consisting solely of ideal reversible processes. The Carnot cycle is such a cycle and it provides an upper limit on the performance of a real cycle operating between the same two thermal reservoirs. Carnot cycle consists of the following 4 reversible processes: 12 Adiabatic compression 23 Isothermal expansion 34 Adiabatic expansion 41 Isothermal compression
- 20. Process 1-2 Reversible Adiabatic Compression: - Gas is compressed very slowly (quasi-equilibrium) so pressure remains uniform throughout the system - Work done on the system: - Heat transfer QH to the gas, temperature stays at TH
- 21. Process 2-3 Reversible Isothermal Expansion - Gas expands slowly, gas cools but as soon as the temperature drops dT it obtains heat from hot reservoir raising the temperature back to TH. Since temperature difference is always dT heat transfer is reversible - Work done by the system - Heat transfer QH to the gas, temperature stays at TH Process 3-4 Reversible Adiabatic Expansion: - Gas expands very slowly (quasi-equilibrium) so pressure remains uniform throughout the system - Work done by the system: Gas temperature decreases from TH to TC Process 4-1 Reversible Isothermal Compression - Work done on the system - Heat transfer QC from the gas, temperature stays at TC
- 22. Carnot cycle on a P-v diagram Net heat transfer per cycle = QH - QC
- 23. Carnot Refrigeration Cycle The Carnot heat-engine cycle is composed of totally reversible processes. The reverse of the heat engine cycle is the Carnot refrigeration cycle. Heat is removed from the cold reservoir and heat is added to the hot reservoir.
- 24. Carnot Principles 1. The efficiency of an irreversible heat engine is always less than the efficiency of a reversible one operating between the same two reservoirs. 2. The efficiencies of all reversible heat engines operating between the same two reservoirs are the same. Violation of either principle results in the violation of K-P Example: Consider two heat engines operating between the same two reservoirs, one reversible and the other irreversible, and each engine is supplied the same amount of heat QH
- 26. Kelvin Temperature Scale Carnot Pr. #2 implies that the efficiency of a reversible cycle is independent of the working fluid and depends only on the temperature of the reservoir ηR = g(TH,TC) Consider three reversible engines A, B and D. Where A and D draw heat Q1 from the same reservoir at T1 Engines A and B can be combined into one reversible engine C
- 27. For each engine we can state (Q4 = Q3) Since left hand side (LHS) is independent of T2 the RHS must also be independent of T2 This condition is satisfied only if: Lord Kelvin proposed taking φ(T) = T, so that
- 28. The thermal efficiency of a reversible heat engine is The thermal efficiency of a reversible refrigerator is This is known as the Carnot Efficiency, it represents the highest possible efficiency for an engine operating between two reservoirs at TC and TH Use reversible heat engine to measure temperature of an object at temperature T on Kelvin scale by measuring QH and QC
- 29. Gas Power Cycles Deal with systems that produce power in which the working fluid remains a gas throughout the cycle, i.e., no change in phase. Internal Combustion (IC) Engine There are two types of reciprocating engines: 1) Spark ignition – Otto cycle 2) Compression – Diesel cycle Terminology:
- 30. Four stroke Spark Ignition Engine: Cycle consists of four distinct strokes (processes) A complete cycle (4 strokes) requires two revolutions of the crank shaft
- 31. A comprehensive study of IC engines requires consideration of the details of the air intake, combustion, and exhaust processes (MECH 435 – 4th year elective) Thermodynamic Air-Standard Analysis Used to perform elementary analyses of IC engines. Simplifications to the real cycle include: 1) Fixed amount of air (ideal gas) for working fluid 2) Combustion process replaced by constant volume heat addition with piston at TDC 3) Intake and exhaust not considered, cycle completed with constant volume heat removal with piston at BDC 4) All processes considered internally reversible Air-Standard Otto cycle Process 1 2 Isentropic compression Process 2 3 Constant volume heat addition Process 3 4 Isentropic expansion Process 4 1 Constant volume heat removal