Titration or titrimetry
•
18 gefällt mir•9,656 views
These slides represents the analytical techniques used for analysis of unknown samples.
Melden
Teilen
Melden
Teilen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Analytical chemistry, errors, significant figures & Calibration

Analytical chemistry, errors, significant figures & Calibration
B.S 4- Class 1-Introduction to analytical chemistry

B.S 4- Class 1-Introduction to analytical chemistry
Ähnlich wie Titration or titrimetry
Ähnlich wie Titration or titrimetry (20)
Titrimetric analysis of drugs based on 1) neutralization 2)Hydrolysis 3)Oxid...

Titrimetric analysis of drugs based on 1) neutralization 2)Hydrolysis 3)Oxid...
Kürzlich hochgeladen
🌹Attapur⬅️ Vip Call Girls Hyderabad 📱9352852248 Book Well Trand Call Girls In Hyderabad Escorts Service
Escorts Service Available
Whatsapp Chaya ☎️ : [+91-9352852248 ]
Escorts Service Hyderabad are always ready to make their clients happy. Their exotic looks and sexy personalities are sure to turn heads. You can enjoy with them, including massages and erotic encounters.#P12Our area Escorts are young and sexy, so you can expect to have an exotic time with them. They are trained to satiate your naughty nerves and they can handle anything that you want. They are also intelligent, so they know how to make you feel comfortable and relaxed
SERVICE ✅ ❣️
⭐➡️HOT & SEXY MODELS // COLLEGE GIRLS HOUSE WIFE RUSSIAN , AIR HOSTES ,VIP MODELS .
AVAILABLE FOR COMPLETE ENJOYMENT WITH HIGH PROFILE INDIAN MODEL AVAILABLE HOTEL & HOME
★ SAFE AND SECURE HIGH CLASS SERVICE AFFORDABLE RATE
★
SATISFACTION,UNLIMITED ENJOYMENT.
★ All Meetings are confidential and no information is provided to any one at any cost.
★ EXCLUSIVE PROFILes Are Safe and Consensual with Most Limits Respected
★ Service Available In: - HOME & HOTEL Star Hotel Service .In Call & Out call
SeRvIcEs :
★ A-Level (star escort)
★ Strip-tease
★ BBBJ (Bareback Blowjob)Receive advanced sexual techniques in different mode make their life more pleasurable.
★ Spending time in hotel rooms
★ BJ (Blowjob Without a Condom)
★ Completion (Oral to completion)
★ Covered (Covered blowjob Without condom
★ANAL SERVICES.
🌹Attapur⬅️ Vip Call Girls Hyderabad 📱9352852248 Book Well Trand Call Girls In...

🌹Attapur⬅️ Vip Call Girls Hyderabad 📱9352852248 Book Well Trand Call Girls In...Call Girls In Delhi Whatsup 9873940964 Enjoy Unlimited Pleasure
Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Escort ServiceModels Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...

Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...GENUINE ESCORT AGENCY
Model Call Girl Services in Delhi reach out to us at 🔝 9953056974 🔝✔️✔️
Our agency presents a selection of young, charming call girls available for bookings at Oyo Hotels. Experience high-class escort services at pocket-friendly rates, with our female escorts exuding both beauty and a delightful personality, ready to meet your desires. Whether it's Housewives, College girls, Russian girls, Muslim girls, or any other preference, we offer a diverse range of options to cater to your tastes.
We provide both in-call and out-call services for your convenience. Our in-call location in Delhi ensures cleanliness, hygiene, and 100% safety, while our out-call services offer doorstep delivery for added ease.
We value your time and money, hence we kindly request pic collectors, time-passers, and bargain hunters to refrain from contacting us.
Our services feature various packages at competitive rates:
One shot: ₹2000/in-call, ₹5000/out-call
Two shots with one girl: ₹3500/in-call, ₹6000/out-call
Body to body massage with sex: ₹3000/in-call
Full night for one person: ₹7000/in-call, ₹10000/out-call
Full night for more than 1 person: Contact us at 🔝 9953056974 🔝. for details
Operating 24/7, we serve various locations in Delhi, including Green Park, Lajpat Nagar, Saket, and Hauz Khas near metro stations.
For premium call girl services in Delhi 🔝 9953056974 🔝. Thank you for considering us!Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X79953056974 Low Rate Call Girls In Saket, Delhi NCR
Kürzlich hochgeladen (20)
💕SONAM KUMAR💕Premium Call Girls Jaipur ↘️9257276172 ↙️One Night Stand With Lo...

💕SONAM KUMAR💕Premium Call Girls Jaipur ↘️9257276172 ↙️One Night Stand With Lo...
Call Girls Madurai Just Call 9630942363 Top Class Call Girl Service Available

Call Girls Madurai Just Call 9630942363 Top Class Call Girl Service Available
Andheri East ) Call Girls in Mumbai Phone No 9004268417 Elite Escort Service ...

Andheri East ) Call Girls in Mumbai Phone No 9004268417 Elite Escort Service ...
Call Girls Service Jaipur {9521753030} ❤️VVIP RIDDHI Call Girl in Jaipur Raja...

Call Girls Service Jaipur {9521753030} ❤️VVIP RIDDHI Call Girl in Jaipur Raja...
🌹Attapur⬅️ Vip Call Girls Hyderabad 📱9352852248 Book Well Trand Call Girls In...

🌹Attapur⬅️ Vip Call Girls Hyderabad 📱9352852248 Book Well Trand Call Girls In...
Independent Call Girls In Jaipur { 8445551418 } ✔ ANIKA MEHTA ✔ Get High Prof...

Independent Call Girls In Jaipur { 8445551418 } ✔ ANIKA MEHTA ✔ Get High Prof...
💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...

💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...
Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available

Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available
Call Girls Kolkata Kalikapur 💯Call Us 🔝 8005736733 🔝 💃 Top Class Call Girl Se...

Call Girls Kolkata Kalikapur 💯Call Us 🔝 8005736733 🔝 💃 Top Class Call Girl Se...
Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...

Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...
Premium Call Girls In Jaipur {8445551418} ❤️VVIP SEEMA Call Girl in Jaipur Ra...

Premium Call Girls In Jaipur {8445551418} ❤️VVIP SEEMA Call Girl in Jaipur Ra...
Andheri East ^ (Genuine) Escort Service Mumbai ₹7.5k Pick Up & Drop With Cash...

Andheri East ^ (Genuine) Escort Service Mumbai ₹7.5k Pick Up & Drop With Cash...
Low Rate Call Girls Bangalore {7304373326} ❤️VVIP NISHA Call Girls in Bangalo...

Low Rate Call Girls Bangalore {7304373326} ❤️VVIP NISHA Call Girls in Bangalo...
Call Girls Mumbai Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Mumbai Just Call 8250077686 Top Class Call Girl Service Available
Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...

Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...
Call Girls Amritsar Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Amritsar Just Call 8250077686 Top Class Call Girl Service Available
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7
Call Girls Service Jaipur {8445551418} ❤️VVIP BHAWNA Call Girl in Jaipur Raja...

Call Girls Service Jaipur {8445551418} ❤️VVIP BHAWNA Call Girl in Jaipur Raja...
Coimbatore Call Girls in Coimbatore 7427069034 genuine Escort Service Girl 10...

Coimbatore Call Girls in Coimbatore 7427069034 genuine Escort Service Girl 10...
9630942363 Genuine Call Girls In Ahmedabad Gujarat Call Girls Service

9630942363 Genuine Call Girls In Ahmedabad Gujarat Call Girls Service
Titration or titrimetry
- 2. - Rishabh Sharma (M.Sc. ES) CEEES Department Deen bandhu Chhotu ram University of Science and Technology (DCRUST), Murthal, Sonepat Haryana.
- 3. CONTENTS • Introduction • Principle of titration • Terms used in titration • Concentration Terms • Titrimetric calculation • Titrimetric apparatus • Types of titration • Fields of use • Advantages of titration
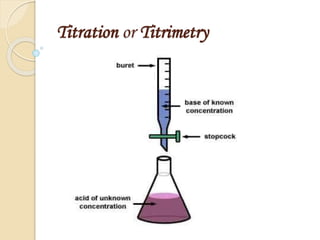
- 4. Introduction A technique for determining the concentration of a solution by measuring the volume of one solution needed to completely react with another solution. Titration process involves addition of solution of known conc. from burette to the measured volume of analyte.
- 5. Principle of titration Principle of titration:- It is based on the complete chemical reaction between the analyte and the reagent (titrant) of known concentration. Analyte + Titrant → Product
- 6. Terms used in titration Analyte Analyte:- The solution of unknown concentration but known volume. Titrant:- The solution of known concentration.
- 7. Standard solution:- A solution of known concentration is called the standard solution. Types of standard solution:- 1) Primary standard:- It has certain properties: (a)Extremely pure. (b)Highly stable. (c) Can be weighed easily. For e.g. Na2CO3.
- 8. 2) Secondary standard:- It has certain properties:- (a) Less pure than primary standard. (b) Less stable than primary standard. (c) Can not be weighed easily. For e.g. NaOH, HCl
- 9. Equivalence Point Equivalence Point:- Point where the amount of two reactants are just equivalent . End point:- The point at which the reaction seems to be complete, this point is usually seen with the help of indicator.
- 10. Indicator Indicator:- An auxiliary substance which helps in the usual detection of the completion of the titration process at the end point. For examples:- Methyl orange, Phenolphthalein, Cresol red, Thymol blue.
- 11. Concentration Terms Concentration Terms:- The concentration of standard solutions (titrants) are generally expressed in units of either molarity (CM, or M) or normality (CN, or N). Molarity (M):-It is the number of moles of a solute dissolved per liter of the solution. Normality(N):- It is the gram equivalent weight of solute dissolved per litre of solution. Molality(m):- It is the number of moles of
- 12. Titrimetric calculation Titrimetric calculation:- It is based on the following law of equivalence:- NaVa = NsVs or MaVa = MsVs Where, Na is the normality of analyte. Va is the volume of the analyte. Ns is the normality of standard solution. Vs is the volume of standard solution used. Ma is the molarity of analyte. Ms is the molarity of standard solution.
- 14. Types of titration Types of titration:- 1.Acid-base titrations 2.Complexometric titrations 3.Redox titrations 4.Precipitation titrations
- 15. Acid – base Titration(neutralization) Acid base Titration( neutralization):- A sample of unknown concentration of acid is estimated with a known concentrated base or vice-verse. acid + base → water + salt HCl + NaOH ------> H2O + NaCl
- 16. Complexometric Titrations Complexometric Titrations:- As the name indicates, the end point is seems by formation of a complex molecule. Here titrant and titrand react to form a complex till end point is reached. Once complex is formed, the complex is stable and not further reaction takes place. Ca+2 + EDTA-4 ------> CaEDTA-2 Mg In + EDTA-4 ------> MgEDTA-2 + -2
- 17. Redox titration Redox titration:- Redox titration is based on the redox reaction (oxidation-reduction) between analyte and titrant. For example:- 6 Fe2+ + 14 H+ + Cr2O7 2- => 6 Fe3+ + 2Cr3+ + 7 H2O MnO4 –(Purple Pink) + 8 H+ + 5 Fe2+ ==>Mn2+(colorless) + 5 Fe3+ + 4 H2O
- 18. Precipitation titrations Precipitation titrations:- The titrations which are based on the formation of insoluble precipitates, when the solutions of two reacting substances are brought in contact with each other, are called Precipitation titration. Ag+(aq)+ Cl−(aq)⇌ AgCl(s)
- 19. Fields of use Fields of use:- Titration is a widely applied analytical technique. Some areas where titration is used are given below:- Agriculture, Oil Industry, Chemical industry, Pharmaceuticals, Food Industry.
- 20. Advantages of titration There are several reasons why titration is used in laboratories worldwide:- 1)Titration is an established analytical technique. 2)It is fast 3) It is a very accurate and precise technique. 4) Titration offers a good price/performance ratio as compared to more sophisticated techniques. 5) It can be used by low-skilled and low- trained operators.