New Horizons for Advanced Endometrial Cancer Treatment: Utilizing Innovative Immunotherapies and Other Novel Approaches
•
0 gefällt mir•101 views
Chair, Shannon N. Westin, MD, MPH, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/NCPD activity titled “New Horizons for Advanced Endometrial Cancer Treatment: Utilizing Innovative Immunotherapies and Other Novel Approaches.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD information, and to apply for credit, please visit us at https://bit.ly/3ecJ3Tk. CME/MOC/NCPD credit will be available until February 6, 2024.
Melden
Teilen
Melden
Teilen
Downloaden Sie, um offline zu lesen
Empfohlen
Edward B. Garon, MD, MS, Jamie E. Chaft, MD, and Matthew D. Hellmann, MD, prepared useful Practice Aids pertaining to lung cancer management for this CME/MOC/CE activity titled "Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Cancer Continuum: State of the Science and Implications for Practice." For the full presentation, monograph, complete CME/MOC/CE information, and to apply for credit, please visit us at http://bit.ly/2ATq0qp. CME/MOC/CE credit will be available until November 21, 2019.Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Ca...

Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Ca...PVI, PeerView Institute for Medical Education
James T. Kenney, RPh, MBA, and Michael B. Atkins, MD, prepared useful Practice Aids pertaining to cancer immunotherapies for this CME/MOC/CE/CPE activity titled "Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in Managed Care Settings: Navigating the Complexities of Value Assessment & Cost Optimization in the Era of Immuno-Oncology." For the full presentation, monograph, complete CME/MOC/CE/CPE information, and to apply for credit, please visit us at http://bit.ly/2Er15gR. CME/MOC/CE/CPE credit will be available until December 23, 2019.Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in M...

Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in M...PVI, PeerView Institute for Medical Education
Daniel Pallin, MD, MPH, and Douglas B. Johnson, MD, MSCI, prepared useful practice aids pertaining to immune-related adverse events for this CME/MOC/CE activity titled "Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Cancer Immunotherapy–Related Adverse Effects in the Emergency Department." For the full presentation, monograph, complete CME/MOC/CE information, and to apply for credit, please visit us at http://bit.ly/2PRv8Ul. CME/MOC/CE credit will be available until November 20, 2019.Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Ca...

Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Ca...PVI, PeerView Institute for Medical Education
Co-Chairs, Nasser Altorki, MD, and Jonathan D. Spicer, MD, PhD, FRCSC, prepared useful Practice Aids pertaining to NSCLC for this CME/MOC activity titled “Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSCLC Help Break the Stalled Cycle of Poor Outcomes?” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/3m1OV2m. CME/MOC credit will be available until February 27, 2023.Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSC...

Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSC...PVI, PeerView Institute for Medical Education
Empfohlen
Edward B. Garon, MD, MS, Jamie E. Chaft, MD, and Matthew D. Hellmann, MD, prepared useful Practice Aids pertaining to lung cancer management for this CME/MOC/CE activity titled "Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Cancer Continuum: State of the Science and Implications for Practice." For the full presentation, monograph, complete CME/MOC/CE information, and to apply for credit, please visit us at http://bit.ly/2ATq0qp. CME/MOC/CE credit will be available until November 21, 2019.Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Ca...

Improving Patient Outcomes With Cancer Immunotherapies Throughout the Lung Ca...PVI, PeerView Institute for Medical Education
James T. Kenney, RPh, MBA, and Michael B. Atkins, MD, prepared useful Practice Aids pertaining to cancer immunotherapies for this CME/MOC/CE/CPE activity titled "Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in Managed Care Settings: Navigating the Complexities of Value Assessment & Cost Optimization in the Era of Immuno-Oncology." For the full presentation, monograph, complete CME/MOC/CE/CPE information, and to apply for credit, please visit us at http://bit.ly/2Er15gR. CME/MOC/CE/CPE credit will be available until December 23, 2019.Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in M...

Incorporating Cancer Immunotherapies Into the Oncology Treatment Arsenal in M...PVI, PeerView Institute for Medical Education
Daniel Pallin, MD, MPH, and Douglas B. Johnson, MD, MSCI, prepared useful practice aids pertaining to immune-related adverse events for this CME/MOC/CE activity titled "Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Cancer Immunotherapy–Related Adverse Effects in the Emergency Department." For the full presentation, monograph, complete CME/MOC/CE information, and to apply for credit, please visit us at http://bit.ly/2PRv8Ul. CME/MOC/CE credit will be available until November 20, 2019.Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Ca...

Emergency Medicine and Immuno-Oncology Intersect: Recognizing and Managing Ca...PVI, PeerView Institute for Medical Education
Co-Chairs, Nasser Altorki, MD, and Jonathan D. Spicer, MD, PhD, FRCSC, prepared useful Practice Aids pertaining to NSCLC for this CME/MOC activity titled “Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSCLC Help Break the Stalled Cycle of Poor Outcomes?” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/3m1OV2m. CME/MOC credit will be available until February 27, 2023.Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSC...

Can the Addition of Immunotherapy to Multimodal Management of Stage I-III NSC...PVI, PeerView Institute for Medical Education
Chair, David M. O'Malley, MD, Ana Oaknin, MD, PhD, and Matthew A. Powell, MD, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/AAPA activity titled “Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evidence Into Meaningful Improvements in Patient Outcomes Across the Disease Continuum.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA information, and to apply for credit, please visit us at https://bit.ly/40bmalK. CME/MOC/AAPA credit will be available until July 3, 2024.Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...

Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...PVI, PeerView Institute for Medical Education
Roy H. Decker, MD, PhD, and Sarah B. Goldberg, MD, MPH, prepared useful practice aids pertaining to lung cancer for this CME activity titled "The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Practicalities of Integrating Checkpoint Inhibition Into the Multimodal Treatment Arsenal." For the full presentation, monograph, complete CME information, and to apply for credit, please visit us at http://bit.ly/2PU3iaZ. CME credit will be available until December 6, 2019.The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...

The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...PVI, PeerView Institute for Medical Education
Chair and Presenters Sumanta Kumar Pal, MD, FASCO, Prof. Laurence Albiges, MD, PhD, and David F. McDermott, MD, prepared useful Practice Aids pertaining to renal cell carcinoma for this CME/MOC/AAPA activity titled “Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Across Treatment Settings.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA information, and to apply for credit, please visit us at https://bit.ly/3LtPuyF. CME/MOC/AAPA credit will be available until December 10, 2024.Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...

Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...PVI, PeerView Institute for Medical Education
Chair Jamie Carroll, APRN, CNP, MSN, discusses breast cancer in this NCPD/ILNA/AAPA activity titled “Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy in TNBC and HR+, HER2- Breast Cancer: Best Practices for Adverse Event Management and Patient Education.” For the full presentation, downloadable Practice Aids, and complete NCPD/ILNA/AAPA information, and to apply for credit, please visit us at https://bit.ly/3SdnvWt. NCPD/ILNA/AAPA credit will be available until May 8, 2025.Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...

Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...PVI, PeerView Institute for Medical Education
Chair Jonathan A. Bernstein, MD, discusses chronic spontaneous urticaria in this CME activity titled “BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Treatment.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3P0cnvi. CME credit will be available until May 6, 2025.BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...

BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, prepared useful Practice Aids pertaining to hypertrophic cardiomyopathy for this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, discuss hypertrophic cardiomyopathy in this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Chair A. Michael Lincoff, MD, discusses obesity in this CME activity titled “Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Weight Loss Pharmacotherapy.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3KAO98K. CME credit will be available until April 25, 2025.Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...

Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...PVI, PeerView Institute for Medical Education
Co-Chairs and Planners Saakshi Khattri, MBBS, MD, FAAD, FACR, Marla Dubinsky, MD, Emma Guttman-Yassky, MD, PhD, and Alexis Ogdie, MD, MSCE, discuss immune-mediated inflammatory diseases in this CME/AAPA activity titled “Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Diseases: Addressing Shared Pathophysiology With JAK Inhibitors.” For the full presentation, downloadable Practice Aids, and complete CME/AAPA information, and to apply for credit, please visit us at https://bit.ly/3JhsIZ7. CME/AAPA credit will be available until April 24, 2025.Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...

Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...PVI, PeerView Institute for Medical Education
Co-Chairs Alicia K. Morgans, MD, MPH, and Neal D. Shore, MD, FACS, discuss prostate cancer in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “Treatment Advances and Individualized Therapeutic Strategies in Prostate Cancer: Expert Insights on Key Evidence, Practical Tips for Personalized Therapy, and Clinical Integration Approaches.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SQrJ6G. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 24, 2025.Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...

Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...PVI, PeerView Institute for Medical Education
Co-Chairs Prof. Nicolas Girard, MD, PhD, and Aaron Lisberg, MD, discuss NSCLC in this CME/MOC/NCPD/AAPA/IPCE activity titled “Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Cancer: Unveiling Potential, Shaping Tomorrow.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3RmX3dU. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 24, 2025.Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...

Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...PVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, prepared useful Practice Aids pertaining to chronic rhinosinusitis with nasal polyps for this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Weitere ähnliche Inhalte
Ähnlich wie New Horizons for Advanced Endometrial Cancer Treatment: Utilizing Innovative Immunotherapies and Other Novel Approaches
Chair, David M. O'Malley, MD, Ana Oaknin, MD, PhD, and Matthew A. Powell, MD, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/AAPA activity titled “Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evidence Into Meaningful Improvements in Patient Outcomes Across the Disease Continuum.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA information, and to apply for credit, please visit us at https://bit.ly/40bmalK. CME/MOC/AAPA credit will be available until July 3, 2024.Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...

Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...PVI, PeerView Institute for Medical Education
Roy H. Decker, MD, PhD, and Sarah B. Goldberg, MD, MPH, prepared useful practice aids pertaining to lung cancer for this CME activity titled "The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Practicalities of Integrating Checkpoint Inhibition Into the Multimodal Treatment Arsenal." For the full presentation, monograph, complete CME information, and to apply for credit, please visit us at http://bit.ly/2PU3iaZ. CME credit will be available until December 6, 2019.The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...

The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...PVI, PeerView Institute for Medical Education
Chair and Presenters Sumanta Kumar Pal, MD, FASCO, Prof. Laurence Albiges, MD, PhD, and David F. McDermott, MD, prepared useful Practice Aids pertaining to renal cell carcinoma for this CME/MOC/AAPA activity titled “Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Across Treatment Settings.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA information, and to apply for credit, please visit us at https://bit.ly/3LtPuyF. CME/MOC/AAPA credit will be available until December 10, 2024.Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...

Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...PVI, PeerView Institute for Medical Education
Ähnlich wie New Horizons for Advanced Endometrial Cancer Treatment: Utilizing Innovative Immunotherapies and Other Novel Approaches (13)
Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...

Endometrial Cancer Care in the Age of Immunotherapy: Translating Clinical Evi...
PD1PDL1 Pathway and its inhibitors for slideshare.pptx

PD1PDL1 Pathway and its inhibitors for slideshare.pptx
The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...

The Era of Immunotherapy in Stage III NSCLC: Exploring the Evidence and Pract...
Managing Immune-Related Adverse Events to Ensure Optimal Cancer Immunotherapy...

Managing Immune-Related Adverse Events to Ensure Optimal Cancer Immunotherapy...
Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...

Leveling Up Our RCC Care Strategy: Real-World Translation of Key Evidence Acr...
Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...

Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...
Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...

Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...
Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...

Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...
Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...

Clinic Correlation and Prognostic Value of P4HB and GRP78 Expression in Gastr...
NON-INVASIVE BIOMARKERS FOR MONITORING THE IMMUNOTHERAPEUTIC RESPONSE TO CANCER 

NON-INVASIVE BIOMARKERS FOR MONITORING THE IMMUNOTHERAPEUTIC RESPONSE TO CANCER
Surviving and Thriving with Gynecologic Cancer - 9.29.18

Surviving and Thriving with Gynecologic Cancer - 9.29.18
Mehr von PVI, PeerView Institute for Medical Education
Chair Jamie Carroll, APRN, CNP, MSN, discusses breast cancer in this NCPD/ILNA/AAPA activity titled “Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy in TNBC and HR+, HER2- Breast Cancer: Best Practices for Adverse Event Management and Patient Education.” For the full presentation, downloadable Practice Aids, and complete NCPD/ILNA/AAPA information, and to apply for credit, please visit us at https://bit.ly/3SdnvWt. NCPD/ILNA/AAPA credit will be available until May 8, 2025.Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...

Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...PVI, PeerView Institute for Medical Education
Chair Jonathan A. Bernstein, MD, discusses chronic spontaneous urticaria in this CME activity titled “BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Treatment.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3P0cnvi. CME credit will be available until May 6, 2025.BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...

BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, prepared useful Practice Aids pertaining to hypertrophic cardiomyopathy for this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, discuss hypertrophic cardiomyopathy in this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Chair A. Michael Lincoff, MD, discusses obesity in this CME activity titled “Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Weight Loss Pharmacotherapy.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3KAO98K. CME credit will be available until April 25, 2025.Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...

Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...PVI, PeerView Institute for Medical Education
Co-Chairs and Planners Saakshi Khattri, MBBS, MD, FAAD, FACR, Marla Dubinsky, MD, Emma Guttman-Yassky, MD, PhD, and Alexis Ogdie, MD, MSCE, discuss immune-mediated inflammatory diseases in this CME/AAPA activity titled “Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Diseases: Addressing Shared Pathophysiology With JAK Inhibitors.” For the full presentation, downloadable Practice Aids, and complete CME/AAPA information, and to apply for credit, please visit us at https://bit.ly/3JhsIZ7. CME/AAPA credit will be available until April 24, 2025.Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...

Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...PVI, PeerView Institute for Medical Education
Co-Chairs Alicia K. Morgans, MD, MPH, and Neal D. Shore, MD, FACS, discuss prostate cancer in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “Treatment Advances and Individualized Therapeutic Strategies in Prostate Cancer: Expert Insights on Key Evidence, Practical Tips for Personalized Therapy, and Clinical Integration Approaches.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SQrJ6G. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 24, 2025.Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...

Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...PVI, PeerView Institute for Medical Education
Co-Chairs Prof. Nicolas Girard, MD, PhD, and Aaron Lisberg, MD, discuss NSCLC in this CME/MOC/NCPD/AAPA/IPCE activity titled “Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Cancer: Unveiling Potential, Shaping Tomorrow.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3RmX3dU. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 24, 2025.Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...

Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...PVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, prepared useful Practice Aids pertaining to chronic rhinosinusitis with nasal polyps for this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, discuss chronic rhinosinusitis with nasal polyps in this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, R. Donald Harvey, PharmD, BCOP, FCCP, FHOPA, FASCO, Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, and James Davis, PharmD, BCOP, discuss multiple myeloma in this CME/CPE/IPCE activity titled “Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering Effective Therapy With Antibody Platforms.” For the full presentation, downloadable Practice Aids, and complete CME/CPE/IPCE information, and to apply for credit, please visit us at https://bit.ly/4aa0iMX. CME/CPE/IPCE credit will be available until May 2, 2025.Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...

Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...PVI, PeerView Institute for Medical Education
Co-Chairs, Carlos G. Romo, MD, and Aimee Sato, MD, discuss Neurofibromatosis in this CME/MOC activity titled “Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & Multimodal Care for NF1 pNF and Other Tumors.” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/3SZRz8p. CME/MOC credit will be available until May 2, 2025.Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...

Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, discuss endometrial cancer in this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Bradley J. Monk, MD, FACS, FACOG, Kathleen N. Moore, MD, MS, and Ana Oaknin, MD, PhD, discuss gynecologic cancers in this CME/MOC/NCPD/AAPA/IPCE activity titled “Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Implementation Strategies, and Patient Care.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4a56tly. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 16, 2025.Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...

Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, prepared useful Practice Aids pertaining to lung cancer for this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, discuss lung cancer screening in this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, prepared useful Practice Aids pertaining to CAR-T therapy for this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, discuss CAR-T therapy in this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Co-Chairs Lipika Goyal, MD, MPhil, and Riad Salem, MD, MBA, discuss HCC in this CME activity titled “The Convergence of Interventional Radiologists and Oncologists in HCC: Shared Decision-Making and Care Coordination at the Center of Personalized Care Across the Disease Continuum.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/48BAasz. CME credit will be available until April 26, 2025.The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...

The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...PVI, PeerView Institute for Medical Education
Mehr von PVI, PeerView Institute for Medical Education (20)
Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...

Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...
BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...

BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...
Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...
Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...
Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...

Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...
Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...

Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...
Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...

Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...
Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...

Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...
Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into Practice
Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into Practice
Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...

Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...
Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...

Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...
Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...
Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...
Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...

Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...
Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...
Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...
Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...
Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...
The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...

The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...
Kürzlich hochgeladen
Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Escort ServiceModels Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...

Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...GENUINE ESCORT AGENCY
Model Call Girl Services in Delhi reach out to us at 🔝 9953056974 🔝✔️✔️
Our agency presents a selection of young, charming call girls available for bookings at Oyo Hotels. Experience high-class escort services at pocket-friendly rates, with our female escorts exuding both beauty and a delightful personality, ready to meet your desires. Whether it's Housewives, College girls, Russian girls, Muslim girls, or any other preference, we offer a diverse range of options to cater to your tastes.
We provide both in-call and out-call services for your convenience. Our in-call location in Delhi ensures cleanliness, hygiene, and 100% safety, while our out-call services offer doorstep delivery for added ease.
We value your time and money, hence we kindly request pic collectors, time-passers, and bargain hunters to refrain from contacting us.
Our services feature various packages at competitive rates:
One shot: ₹2000/in-call, ₹5000/out-call
Two shots with one girl: ₹3500/in-call, ₹6000/out-call
Body to body massage with sex: ₹3000/in-call
Full night for one person: ₹7000/in-call, ₹10000/out-call
Full night for more than 1 person: Contact us at 🔝 9953056974 🔝. for details
Operating 24/7, we serve various locations in Delhi, including Green Park, Lajpat Nagar, Saket, and Hauz Khas near metro stations.
For premium call girl services in Delhi 🔝 9953056974 🔝. Thank you for considering us!Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X79953056974 Low Rate Call Girls In Saket, Delhi NCR
Kürzlich hochgeladen (20)
Jogeshwari ! Call Girls Service Mumbai - 450+ Call Girl Cash Payment 90042684...

Jogeshwari ! Call Girls Service Mumbai - 450+ Call Girl Cash Payment 90042684...
Call Girls in Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service Avai...

Call Girls in Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service Avai...
Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available

Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available
Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...

Models Call Girls In Hyderabad 9630942363 Hyderabad Call Girl & Hyderabad Esc...
Premium Call Girls In Jaipur {8445551418} ❤️VVIP SEEMA Call Girl in Jaipur Ra...

Premium Call Girls In Jaipur {8445551418} ❤️VVIP SEEMA Call Girl in Jaipur Ra...
Russian Call Girls Service Jaipur {8445551418} ❤️PALLAVI VIP Jaipur Call Gir...

Russian Call Girls Service Jaipur {8445551418} ❤️PALLAVI VIP Jaipur Call Gir...
Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...

Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...
Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time

Top Quality Call Girl Service Kalyanpur 6378878445 Available Call Girls Any Time
VIP Hyderabad Call Girls Bahadurpally 7877925207 ₹5000 To 25K With AC Room 💚😋

VIP Hyderabad Call Girls Bahadurpally 7877925207 ₹5000 To 25K With AC Room 💚😋
Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...

Premium Bangalore Call Girls Jigani Dail 6378878445 Escort Service For Hot Ma...
Mumbai ] (Call Girls) in Mumbai 10k @ I'm VIP Independent Escorts Girls 98333...![Mumbai ] (Call Girls) in Mumbai 10k @ I'm VIP Independent Escorts Girls 98333...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Mumbai ] (Call Girls) in Mumbai 10k @ I'm VIP Independent Escorts Girls 98333...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Mumbai ] (Call Girls) in Mumbai 10k @ I'm VIP Independent Escorts Girls 98333...
💕SONAM KUMAR💕Premium Call Girls Jaipur ↘️9257276172 ↙️One Night Stand With Lo...

💕SONAM KUMAR💕Premium Call Girls Jaipur ↘️9257276172 ↙️One Night Stand With Lo...
(Low Rate RASHMI ) Rate Of Call Girls Jaipur ❣ 8445551418 ❣ Elite Models & Ce...

(Low Rate RASHMI ) Rate Of Call Girls Jaipur ❣ 8445551418 ❣ Elite Models & Ce...
Call Girls Varanasi Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Varanasi Just Call 8250077686 Top Class Call Girl Service Available
💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...

💚Call Girls In Amritsar 💯Anvi 📲🔝8725944379🔝Amritsar Call Girl No💰Advance Cash...
Call Girls Madurai Just Call 9630942363 Top Class Call Girl Service Available

Call Girls Madurai Just Call 9630942363 Top Class Call Girl Service Available
Call Girl In Pune 👉 Just CALL ME: 9352988975 💋 Call Out Call Both With High p...

Call Girl In Pune 👉 Just CALL ME: 9352988975 💋 Call Out Call Both With High p...
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Call Girls in Gagan Vihar (delhi) call me [🔝 9953056974 🔝] escort service 24X7
Most Beautiful Call Girl in Bangalore Contact on Whatsapp

Most Beautiful Call Girl in Bangalore Contact on Whatsapp
New Horizons for Advanced Endometrial Cancer Treatment: Utilizing Innovative Immunotherapies and Other Novel Approaches
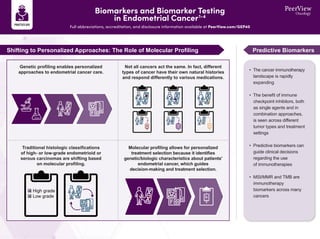
- 1. Shifting to Personalized Approaches: The Role of Molecular Profiling Predictive Biomarkers Genetic profiling enables personalized approaches to endometrial cancer care. Not all cancers act the same. In fact, different types of cancer have their own natural histories and respond differently to various medications. Traditional histologic classifications of high- or low-grade endometrioid or serous carcinomas are shifting based on molecular profiling. Molecular profiling allows for personalized treatment selection because it identifies genetic/biologic characteristics about patients' endometrial cancer, which guides decision-making and treatment selection. High grade Low grade • The cancer immunotherapy landscape is rapidly expanding • The benefit of immune checkpoint inhibitors, both as single agents and in combination approaches, is seen across different tumor types and treatment settings • Predictive biomarkers can guide clinical decisions regarding the use of immunotherapies • MSI/MMR and TMB are immunotherapy biomarkers across many cancers Biomarkers and Biomarker Testing in Endometrial Cancer1-4 Full abbreviations, accreditation, and disclosure information available at PeerView.com/GEP40
- 2. MSI-H and dMMR are considered the same population biologically—both groups of patients can be treated with the same agents • dMMR/MSI-H refers to a group of patients with mismatch repair deficiency • MMRp/MSS refers to a group of patients who are mismatch repair proficient Microsatellite Instability (MSI) MSI is detected through molecular testing Consensus definition: MSI is a condition of genetic hypermutability Characterized by the clustering of mutations in microsatellites typically consisting of repeat length alterations The presence of MSI represents evidence that MMR is not functioning normally, or dMMR MSI-H provides the phenotypic evidence of dMMR Mismatch Repair (MMR) MMR proteins are detected by IHC stain MMR protein complexes (MLH1 + PMS2 and MSH2 + MSH6) detect and correct mistakes during DNA replication Absence or loss of function of one of the four MMR proteins results in MMR deficiency, or dMMR dMMR is the cause of MSI-high, or MSI-H Biomarkers and Biomarker Testing in Endometrial Cancer1-4 Full abbreviations, accreditation, and disclosure information available at PeerView.com/GEP40 1. Kloor M et al. Trends Cancer. 2016;2:121-133. 2. Luchini C et al. Ann Oncol. 2019; 30:1232-1243. 3. Hause RJ et al. Nat Med. 2016;22:1342-1350. 4. Levine DA. Nature. 2013;497:67.
- 3. Managing Immune-Related Adverse Events1-4 Full abbreviations, accreditation, and disclosure information available at PeerView.com/GEP40 Safety Considerations for Immunotherapies 1. Monitor closely for potential irAEs by evaluating blood work including liver enzymes, creatinine, and thyroid function 2. Ask patients about symptoms such as cough, shortness of breath, and diarrhea, which may be signs of pneumonitis or colitis 3. Stay in communication with patients to help mitigate and treat more common AEs such as fatigue, nausea, and anemia Pancreatitis, autoimmune diabetes Colitis Enteritis Encephalitis, aseptic meningitis Thyroiditis, hypothyroidism, hyperthyroidism Dry mouth, mucositis Hypophysitis Uveitis Pneumonitis Thrombocytopenia, anemia Hepatitis Adrenal insufficiency Nephritis Vasculitis Arthralgia Neuropathy Rash, vitiligo Myocarditis Any organ system can be affected; commonly occurring irAEs are pulmonary (pneumonitis), dermatologic (rash, pruritus, blisters, ulcers, vitiligo), gastrointestinal (diarrhea, enterocolitis, transaminitis, hepatitis, pancreatitis), and endocrine (thyroiditis, hypophysitis, adrenal insufficiency) What Is the Spectrum of Potential irAEs? • In general, checkpoint inhibitor therapy should be continued with close monitoring, with the exception of some neurologic, hematologic, and cardiac toxicities Minimal or no symptoms; diagnostic changes only Grade 1 • Hold checkpoint inhibitor therapy for most grade 2 toxicities • Consider resuming immunotherapy when symptoms and/or laboratory values revert to grade 1 or lower • Corticosteroids (initial dose of 0.5-1 mg/kg/d of prednisone or equivalent) may be administered Grade 3 toxicities • Hold checkpoint inhibitor therapy • Initiate high-dose corticosteroids (prednisone 1-2 mg/kg/d or methylprednisolone IV 1-2 mg/kg/d) • If symptoms do not improve with 48-72 hours of high-dose corticosteroids, infliximab may be offered for some toxicities • Taper corticosteroids over the course of at least 4-6 weeks • When symptoms and/or laboratory values revert to grade 1 or lower, rechallenging with immunotherapy may be offered; however, caution is advised, especially in those patients with early-onset irAEs; dose adjustments are not recommended Grade 4 toxicities • In general, permanent discontinuation of checkpoint inhibitor therapy is warranted, with the exception of endocrinopathies that have been controlled by hormone replacement Grade 2 Mild to moderate symptoms Severe or life-threatening symptoms Grades 3/4 Most significant irAEs occur in less than 5% of patients irAE Grading and Managment
- 4. 1. Postow MA et al. N Engl J Med. 2018;378:158-168. 2. Brahmer JR et al. J Clin Oncol. 2018;36:1714-1768. 3. NCCN Clinical Practice Guidelines in Oncology. Management of Immunotherapy-Related Toxicities. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. 4. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. 5. Makker V et al. Oncologist. 2021;26:e1599-e1608. 6. How JA et al. Gynecol Oncol. 2021;162:24-31. Managing Immune-Related Adverse Events5,6 Full abbreviations, accreditation, and disclosure information available at PeerView.com/GEP40 Consult irAE management guidelines (eg, ASCO, NCCN, SITC, ESMO) IO Pruritus Pneumonitis Myocarditis Adrenal crisis TKI Hypertension Taste changes Stomatitis Dyspepsia Cytopenia HFSR Overlapping Rash Diarrhea Hepatitis Hypothyroid AMS IO + TKI Combination Toxicities Determine which therapy is causing the AE in order to plan a management strategy Hold TKI (shorter half-life than checkpoint inhibitor) In certain cases, use appropriate supportive care If symptoms resolve in a few days, TKI was likely the cause Two mechanisms of action result in two sets of AE profiles that are not mutually exclusive PRES Encephalitis
- 5. Numerous Strategies Under Study in Advanced Endometrial Cancer Full abbreviations, accreditation, and disclosure information available at PeerView.com/GEP40 1. https://clinicaltrials.gov. 2. Daly RJ et al. Mol Cancer. 2022;21:189. 3. Bailly C et al. NAR Cancer. 2020;2(1): zcaa002. 4. Vergote IB et al. ESMO 2022. 5. Makker V et al. ASCO 2022. Abstract 5511. 6. Fung HY and Chook YM. Semin Cancer Biol. 2014;27:52-61. 7. Tai YT et al. Leukemia. 2014;28:155-165. • Immune checkpoint inhibitors work by blocking T-cell inhibitory signals, thus removing the brakes on the immune system • The combination of increased mutational load, tumor-infiltrating lymphocytes, and PD-1/PD-L1 expression makes endometrial cancer an ideal target for immunotherapeutic interventions • Exportin 1 (XPO1) is the major nuclear export protein for6 – Tumor suppressor proteins (TSPs), eg, p53, lkB, PTEN, FOXO1 • Inhibition of XPO1 results in6 – Increase in nuclear levels and activation of TSPs – Reduction of oncoprotein levels • Selinexor is an oral selective XPO1 inhibitor – Preclinical data shows reactivation of multiple TSPs (including p53 wild-type) by preventing nuclear export7 IO Monotherapy Combinations XPO1 Inhibition4,5 PD-1/PD-L1 Checkpoint Inhibition - - Without Immunotherapy With Immunotherapy MHC Antigen TCR PD-1 PD-L1 Anti– PD-L1 Anti– PD-1 Tumor cell Tumor escape Inactivation of T Cell Activation of T Cell Elimination of tumor cells • Selected trials1 – Phase 2 KEYNOTE-158 (pembrolizumab) – Phase 1 GARNET (dostarlimab) – Phase 2 PHAEDRA (durvalumab) • Selected trials1 – Phase 3 KEYNOTE-775 (pembrolizumab + lenvatanib) – Phase 3 LEAP-001 (pembrolizumab + lenvatanib) • Selected trials1 – Phase 2 SIGN – Phase 3 SIENDO – Phase 3 XPORT-EC • Angiogenesis and evasion of immune destruction are hallmarks of cancer, supporting the rationale for combining VEGFR TKI and immunotherapies targeting PD‐1/PD-L1 • Targeting TKIs may promote an immune- permissive tumor environment and enhance responses to immune checkpoint inhibitors Cell membrane Nuclear envelope Nuclear pore complex Cytoplasm Nucleus Selinexor Tumor Suppressor Proteins P53 pRb P21 IkB BRCA1 FOXO3a Oncoprotein mRNA eF4E c-Myc Bcl-6 Bcl-2 Cyclin D1 Pim1 MDM2 Glucocorticoid Receptor XPO1 IO + TKI2 IO + Chemo3 • Cytotoxic agents have immunomodulatory effects, providing a rationale for combining PD-1/PD-L1 inhibitors with chemotherapy • Chemotherapy may be synergistic in combination with immunotherapy; its ability to increase tumor immunogenicity may enhance tumor-specific T-cell activation when combined with immune checkpoint blockade • Selected trials1 – Phase 3 NRG-GY018 (pembrolizumab + chemo) – Phase 3 AtTEnd (atezolizumab + chemo) – Phase 3 RUBY (dostarlimab + chemo) – Phase 3 DUO-E (durvalumab + chemo)
