Facilitating Progress in the Treatment of SCLC: How to Optimize the Use of Current Systemic Options and Accelerate the Clinical Transition of Investigational Approaches
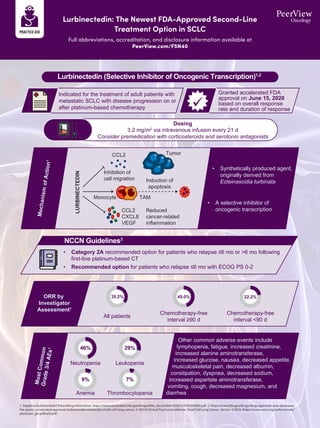
- 1. Dosing 3.2 mg/m2 via intravenous infusion every 21 d Consider premedication with corticosteroids and serotonin antagonists NCCN Guidelines3 Lurbinectedin (Selective Inhibitor of Oncogenic Transcription)1,2 • Category 2A recommended option for patients who relapse ≤6 mo or >6 mo following first-line platinum-based CT • Recommended option for patients who relapse ≤6 mo with ECOG PS 0-2 • Synthetically produced agent, originally derived from Ecteinascidia turbinata • A selective inhibitor of oncogenic transcription Other common adverse events include lymphopenia, fatigue, increased creatinine, increased alanine aminotransferase, increased glucose, nausea, decreased appetite, musculoskeletal pain, decreased albumin, constipation, dyspnea, decreased sodium, increased aspartate aminotransferase, vomiting, cough, decreased magnesium, and diarrhea Indicated for the treatment of adult patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy Granted accelerated FDA approval on June 15, 2020 based on overall response rate and duration of response Neutropenia Leukopenia Anemia Thrombocytopenia M o s t C o m m o n G r a d e 3 / 4 A E s 1 M e c h a n is m o f A c t io n 1 46% 9% 29% 7% ORR by Investigator Assessment1 All patients 35.2% Chemotherapy-free interval ≥90 d 45.0% Chemotherapy-free interval <90 d 22.2% Tumor CCL2 Induction of apoptosis Monocyte CCL2 CXCL8 VEGF Reduced cancer-related inflammation TAM Inhibition of cell migration LURBINECTEDIN Lurbinectedin: The Newest FDA-Approved Second-Line Treatment Option in SCLC Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40 1. Zepzelca (lurbinectedin) Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213702s000lbl.pdf. 2. https://www.fda.gov/drugs/drug-approvals-and-databases/ fda-grants-accelerated-approval-lurbinectedin-metastatic-small-cell-lung-cancer. 3. NCCN Clinical Practice Guidelines. Small Cell Lung Cancer. Version 3.2023. https://www.nccn.org/professionals/ physician_gls/pdf/sclc.pdf.
- 2. Immune Checkpoints • Activated T cells recognize cognate antigen presented by cancer cells TCR triggered negative regulatory receptor PD-1 expressed IFN-γ produced reactive expression of PD-L1 antitumor T-cell responses turned off This negative interaction can be blocked by anti–PD-1 or anti–PD-L1 antibody therapies – Examples of anti–PD-1 antibodies: nivolumab, pembrolizumab, cemiplimab-rwlc – Examples of anti–PD-L1 antibodies: durvalumab, atezolizumab, avelumab • CTLA-4 is a negative regulator of costimulation that is required for initial activation of an antitumor T cell in a lymph node upon recognition of tumor antigen This negative interaction can be blocked by anti–PD-1 or anti–PD-L1 antibody therapies – Examples of anti–CTLA-4 antibodies: ipilimumab, tremelimumab Tumor Microenvironment Lymphoid Tissue Elimination of tumor cells Tumor escape With Immunotherapy Without Immunotherapy Elimination of tumor cells Tumor escape With Immunotherapy Without Immunotherapy • Proteins on T cells or cancer cells that need to be activated/inactivated to start/stop an immune response – Examples include PD-1, PD-L1, CTLA-4 • Serve as “brakes” that help keep immune responses in check and can prevent T-cell response against cancer cells • Can be blocked by immune checkpoint inhibitors – The “brakes” on the immune system are released and T cells are able to attack and kill cancer cells PD-1/PD-L1 Checkpoint Inhibition1 CTLA-4 Checkpoint Inhibition1 The Immunotherapy Era in SCLC: Treatment, Adverse Events, and PD-1/PD-L1 Checkpoint Inhibition Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40
- 3. Indicated in combination with etoposide and either carboplatin or cisplatin, as first-line treatment of adult patients with extensive-stage SCLC Granted FDA approval in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with extensive-stage SCLC on March 27, 2020 Indicated in combination with etoposide and either carboplatin or cisplatin, as first-line treatment of adult patients with ES-SCLC Granted FDA approval in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with ES-SCLC on March 27, 2020 Indicated in combination with etoposide and either carboplatin or cisplatin, as first-line treatment of adult patients with extensive-stage SCLC Granted FDA approval in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with extensive-stage SCLC on March 27, 2020 Indicated in combination with carboplatin and etoposide for first-line treatment of adult patients with ES-SCLC Granted FDA approval in combination with carboplatin and etoposide for first-line treatment of adult patients with ES-SCLC on March 18, 2019 Dosing2 1,500 mg every 3 weeks prior to chemotherapy in combination with etoposide and either carboplatin or cisplatin and then every 4 weeks as a single agent Most Common AEs (All Grades)2 Nausea (34%) Fatigue/ asthenia (32%) Alopecia (31%) Most Common AEs (All Grades)8 Nausea Fatigue/ asthenia Alopecia Diarrhea Constipation Decreased appetite Outcomes From the CASPIAN Trial: A Randomized, Controlled, Open-Label, Phase 3 Trial4-7 Confirmed Objective Response, n (%)a Median Duration of Response, mo (95% CI) Durvalumab + Platinum-etoposide (n = 268) Platinum- etoposide (n = 269) 182 (68) 5.1 (4.9-5.3) 156 (58) 5.1 (4.8-5.3) a Objective response by investigator review per RECIST 1.1 is defined as pts with complete response or partial response on at least one visit (unconfirmed responses); for confirmed responses, a confirmatory scan was required no sooner than 4 weeks after the initial response. Outcomes From the IMpower33 Trial: A Double-Blind, Placebo-Controlled, Phase 3 Trial10 b Assessed in pts in the ITT population who had measurable disease at baseline. Defined as confirmed complete response or partial response by investigator review per RECIST 1.1. c Assessed in pts who had a confirmed objective response. Defined as the time from first occurrence of a documented objective response to the time of disease progression as determined by the investigator (according to RECIST) or death from any cause, whichever occurred first. Dosing for ES-SCLC8 1,200 mg every 3 weeks prior to chemotherapy with carboplatin and etoposide; 840 mg every 2 weeks, 1,200 mg every 3 weeks, or 1,680 mg every 4 weeks following completion of 4 cycles of carboplatin and etoposide Confirmed Objective Response, n (%)b Median Duration of Response, mo (range)c Median Overall Survival, mo (95% CI) Median PFS, mo (95% CI) Atezolizumab (n = 201) Placebo (n = 202) 121 (60.2) 4.2 (1.4-19.5) 12.3 (10.8-15.9) 5.2 (4.4-5.6) 130 (64.4) 3.9 (2.0-16.1) 10.3 (9.3-11.3) 4.3 (4.2-4.5) Durvalumab (PD-L1–Blocking Antibody)2,3 Atezolizumab (PD-L1–Blocking Antibody)8,9 Median Overall Survival, mo (95% CI) Median PFS, mo (95% CI) 12.9 (11.3-14.7) 5.1 (4.7-6.2) 10.5 (9.3-11.2) 5.4 (4.8-6.2) The Immunotherapy Era in SCLC: Treatment, Adverse Events, and PD-1/PD-L1 Checkpoint Inhibition Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40
- 4. Endocrine Hyper- or hypothyroidism Hypophysitis Adrenal insufficiency Diabetes Hepatic Hepatitis Renal Nephritis Dermatologic Rash Pruritus Psoriasis Vitiligo DRESS Stevens-Johnson Hematologic Hemolytic anemia Thrombocytopenia Neutropenia Hemophilia Ocular Uveitis Conjunctivitis Scleritis, episcleritis Blepharitis Retinitis Respiratory Pneumonitis Pleuritis Sarcoid-like granulomatosis Cardiovascular Myocarditis Pericarditis Vasculitis Gastrointestinal Colitis Ileitis Pancreatitis Gastritis Neurologic Neuropathy Guillain-Barré syndrome Myelopathy Encephalitis Myasthenia Musculoskeletal Arthritis Dermatomyositis Prevention Anticipation Treatment Monitoring Detection What Are irAEs? Immune-Related Adverse Events (irAEs)11-14 • Immune checkpoint inhibitors are associated with important clinical benefits, but general immunologic enhancement can also lead to a unique spectrum of immune-related adverse effects • Any organ system can be affected, but more commonly occurring are pulmonary (pneumonitis), dermatologic (rash, pruritus, blisters, ulcers, vitiligo), gastrointestinal (diarrhea, enterocolitis, transaminitis, hepatitis, pancreatitis), and endocrine (thyroiditis, hypophysitis, adrenal insufficiency) irAEs The Immunotherapy Era in SCLC: Treatment, Adverse Events, and PD-1/PD-L1 Checkpoint Inhibition Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40
- 5. How Should irAEs Be Diagnosed and Managed? • In general, immunotherapy should be continued with close monitoring, with the exception of some neurologic, hematologic, and cardiac toxicities Minimal or No Symptoms; Diagnostic Changes Only Grade 1 Mild to Moderate Symptoms Grade 2 • Hold checkpoint inhibitor therapy for most grade 2 toxicities • Consider resuming immunotherapy when symptoms and/or lab values revert to grade 1 • Corticosteroids (initial dose of 0.5-1 mg/kg/day of prednisone or equivalent) may be administered Severe or Life-Threatening Symptoms Grade 3/4 Grade 3 toxicities • Hold checkpoint inhibitor therapy • Initiate high-dose corticosteroids (prednisone 1-2 mg/kg/day or methylprednisolone IV 1-2 mg/kg/day) • If symptoms do not improve within 48-72 hours of high-dose corticosteroid, infliximab may be offered for some toxicities • Taper corticosteroids over the course of at least 4-6 weeks • When symptoms and/or laboratory values revert to grade 1, rechallenging with immunotherapy may be considered; however, caution is advised, especially in those patients with early-onset irAEs; dose adjustments are not recommended Grade 4 toxicities • In general, permanent discontinuation of checkpoint inhibitor therapy is warranted, with the exception of endocrinopathies that have been controlled by hormone replacement irAEs are often diagnosed by exclusion; other causes should be ruled out (including AEs of other therapies used), but immunotherapy- related toxicity should always be included in the differential There should be a high level of suspicion that new symptoms are treatment related; early recognition, evaluation, and treatment of irAEs plus patient education are essential for the best outcome Depending on severity of irAEs, management may require corticosteroid or other immunosuppressive treatment and interruption or discontinuation of therapy If appropriate immunosuppressive treatment is used, patients generally recover from irAEs Use of immunosuppressive therapy to manage irAEs does not appear to impact response to immunotherapy The Immunotherapy Era in SCLC: Treatment, Adverse Events, and PD-1/PD-L1 Checkpoint Inhibition Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40
- 6. How Should Pulmonary irAEs Be Diagnosed and Managed? Hold immunotherapy with radiographic evidence of pneumonitis progression May offer one repeat CT in 3-4 weeks; in patients who have had baseline testing, may offer a repeat spirometry/DLCO in 3-4 weeks May resume immunotherapy with radiographic evidence of improvement or resolution; if no improvement, should treat as grade 2 Monitor patients weekly with history, physical examination, and pulse oximetry; may also offer CXR Grade 3: Severe symptoms requiring hospitalization; involves all lung lobes or >50% of lung parenchyma; limiting self-care Grade 4: Life-threatening respiratory compromise; urgent intervention indicated (intubation) Hold immunotherapy until resolution to grades ≤1 Prednisone 1-2 mg/kg/day and taper by 5-10 mg/week over 4-6 weeks Consider bronchoscopy with BAL Consider empiric antibiotics Monitor patients every 3 days with history, physical examination, and pulse oximetry; consider CXR; if no clinical improvement after 48-72 hours of prednisone, treat as grade 3 Discontinue immunotherapy Empiric antibiotics; methylprednisolone IV 1-2 mg/kg/day; if no improvement after 48 hours, may add infliximab 5 mg/kg, or mycophenolate mofetil IV 1 g 2x/day, or IVIG x 5 days, or cyclophosphamide Taper corticosteroids over 4-6 weeks Pulmonary and infectious disease consults if necessary Bronchoscopy with BAL ± transbronchial biopsy Patients should be hospitalized for further management Pneumonitis: Focal or diffuse inflammation of the lung parenchyma (typically identified on CT imaging) Diagnostic work-up: CXR, CT, pulse oximetry; for grade ≥2, may include infectious work-up Grade 1: Asymptomatic; confined to 1 lobe of lung or <25% of lung parenchyma; clinical or diagnostic observations only Additional considerations Grade 2: Symptomatic; >1 lobe of lung or 25%-50% of lung parenchyma; medical intervention indicated; limiting instrumental ADL • GI and pneumocystis prophylaxis may be offered to patients on prolonged steroid use (>12 weeks) • Consider calcium and vitamin D supplementation with prolonged steroid use • Bronchoscopy + biopsy; if clinical picture is consistent with pneumonitis, no need for biopsy The Immunotherapy Era in SCLC: Treatment, Adverse Events, and PD-1/PD-L1 Checkpoint Inhibition Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40 1. Adapted from: Soularue E et al. Gut. 2018;67:2056-2067. 2. Imfinzi (durvalumab) Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761069s035lbl.pdf. 3. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves- durvalumab-extensive-stage-small-cell-lung-cancer. 4. Paz-Ares L et al. Lancet. 2019;394:1929-1939. 5. Goldman JW et al. Lancet Oncol. 2021;22:51-65. 6. Paz-Ares L et al. ESMO Open. 2022;7:100408. 7. Paz-Ares LG et al. ESMO 2021. Abstract LBA61. 8. Tecentriq (atezolizumab) Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761034s043lbl.pdf. 9. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-extensive-stage-small-cell-lung-cancer. 10. Horn L et al. N Engl J Med. 2018;379:2220-2229. 11. Brahmer JR et al. J Clin Oncol. 2018;36:1714-1786. 12. Postow MA et al. N Engl J Med. 2018;378:158-168. 13. Gordon R et al. Clin J Oncol Nurs. 2017;21(suppl 2):45-52. 14. Champiat S et al. Ann Oncol. 2016;27:559-574.
- 7. SCLC Treatment Algorithm1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/FSN40 1. Created by Aakash Desai, MBBS, MPH. Used with permission from the author. 2. Paz-Ares L et al. ESMO Open. 2022;7:100408. 3. Paz-Ares LG et al. ESMO 2021. Abstract LBA61. SCLC treatment algorithm Cancer confined to one hemithorax and regional lymph nodes? (can be treated with single radiation field) Stage and workup CT abd/pelvis, PET/CT scan (if needed), MRI brain, PFTs, bone marrow biopsy (select cases), mediastinal staging (stage I-IIA) Prophylactic cranial irradiation in patients achieving remission (ALL stages); take into account performance status, comorbidities, and age Meta-analysis: PCI vs no PCI, RR of death HR: 0.84 (P = .01) EXTENSIVE STAGE (stage III or IV disease not eligible for treatment of curative intent) LIMITED STAGE (eligible for treatment of curative intent) Stage I-IIA Pathologic mediastinal staging Stage IIB-IIIC Lobectomy + mediastinal LN dissection >6 mo treatment-free interval? Platinum-based chemotherapy 1L: Chemoimmunotherapy • Carboplatin + etoposide + atezolizumab with maintenance atezolizumab (IMpower133: Chemo + atezo vs chemo mOS: 12.3 vs 10.3 mo [HR, 0.70]) • Carboplatin or cisplatin + etoposide + durvalumab with maintenance durvalumab (CASPIAN: Chemo + durva vs chemo mOS: 12.9 vs 10.5 mo [HR, 0.71])2,3 Platinum-based chemotherapy + concurrent RT (JCOG 9104: Concurrent RT vs sequential RT mOS: 27.2 vs 19.7 mo, HR, 0.70) Best supportive care in consult with palliative medicine If ineligible for immunotherapy, use platinum-based chemotherapy T1–2, N1, M0 T3–4, Nx, M0 Performance status ≤1 or ≥2 due to SCLC Performance status ≥2 due to comorbidities cT1–2, N0, M0 Negative Limited disease Extensive disease Yes No Platinum-based chemotherapy + mediastinal RT pT1-2, N0-1, R0 N2 and/or R1-2 Positive Relapse Yes No 2L: Chemotherapy 2L: Chemotherapy Rechallenge with original platinum- based chemotherapy (preferred) Lurbinectedin PMB1183-B005014: Single arm (ORR: 35%, mDOR: 5.1 mo) Topotecan cyclophosphamide + doxorubicin + vincristine (CAV) Lurbinectedin (preferred) PMB1183-B005014: Single arm (ORR: 35%, mDOR: 5.1 mo) Topotecan (preferred) Trial: Topotecan vs CAV ORR: 23.3% vs 18.3% Clinical trials (preferred) Cyclophosphamide + doxorubicin + vincristine (CAV)
