Solid gas system
•Als PPTX, PDF herunterladen•
0 gefällt mir•460 views
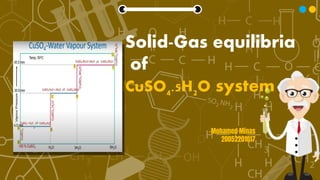
This document describes the solid-gas equilibria of the CuSO4-H2O system. It discusses how copper sulfate forms different hydrates - monohydrate, trihydrate, and pentahydrate - at increasing vapor pressures when water vapor is added at a constant temperature of 55°C. The phase diagram plots vapor pressure against water vapor composition, showing invariant equilibrium lines where two solid phases coexist with vapor, and monovariant lines where a single solid phase coexists with vapor. It also describes the dehydration of copper sulfate pentahydrate upon decreasing vapor pressure, passing through trihydrate and monohydrate before forming anhydrous copper sulfate.
Melden
Teilen
Melden
Teilen
Empfohlen
Key concepts of Geometrical Isomerism useful for the Undergraduate and Postgraduate students of Pharmacy , Chemistry and Post graduates of Pharmaceutical and Medicinal ChemistryGeometrical Isomerism in Olefins

Geometrical Isomerism in OlefinsAteos Foundation of Science Education and Research, Pune, M.S., India
Empfohlen
Key concepts of Geometrical Isomerism useful for the Undergraduate and Postgraduate students of Pharmacy , Chemistry and Post graduates of Pharmaceutical and Medicinal ChemistryGeometrical Isomerism in Olefins

Geometrical Isomerism in OlefinsAteos Foundation of Science Education and Research, Pune, M.S., India
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Chemical dynamics, intro,collision theory by dr. y. s. thakare

Chemical dynamics, intro,collision theory by dr. y. s. thakare
Conformation and Conformation Analysis of Alkanes and Cycloalkanes

Conformation and Conformation Analysis of Alkanes and Cycloalkanes
aromatic,Anti-aromatic and non-aromartic compounds

aromatic,Anti-aromatic and non-aromartic compounds
Ähnlich wie Solid gas system
Ähnlich wie Solid gas system (20)
Phase diagram of a one component system ( water system )

Phase diagram of a one component system ( water system )
Kürzlich hochgeladen
Model Call Girl Services in Delhi reach out to us at 🔝 9953056974 🔝✔️✔️
Our agency presents a selection of young, charming call girls available for bookings at Oyo Hotels. Experience high-class escort services at pocket-friendly rates, with our female escorts exuding both beauty and a delightful personality, ready to meet your desires. Whether it's Housewives, College girls, Russian girls, Muslim girls, or any other preference, we offer a diverse range of options to cater to your tastes.
We provide both in-call and out-call services for your convenience. Our in-call location in Delhi ensures cleanliness, hygiene, and 100% safety, while our out-call services offer doorstep delivery for added ease.
We value your time and money, hence we kindly request pic collectors, time-passers, and bargain hunters to refrain from contacting us.
Our services feature various packages at competitive rates:
One shot: ₹2000/in-call, ₹5000/out-call
Two shots with one girl: ₹3500/in-call, ₹6000/out-call
Body to body massage with sex: ₹3000/in-call
Full night for one person: ₹7000/in-call, ₹10000/out-call
Full night for more than 1 person: Contact us at 🔝 9953056974 🔝. for details
Operating 24/7, we serve various locations in Delhi, including Green Park, Lajpat Nagar, Saket, and Hauz Khas near metro stations.
For premium call girl services in Delhi 🔝 9953056974 🔝. Thank you for considering us!Call Now ≽ 9953056974 ≼🔝 Call Girls In New Ashok Nagar ≼🔝 Delhi door step de...

Call Now ≽ 9953056974 ≼🔝 Call Girls In New Ashok Nagar ≼🔝 Delhi door step de...9953056974 Low Rate Call Girls In Saket, Delhi NCR
Booking open Available Pune Call Girls Koregaon Park 6297143586 Call Hot Indian Girls Waiting For You To Fuck
Booking Contact Details
WhatsApp Chat: +91-6297143586
pune Escort Service includes providing maximum physical satisfaction to their clients as well as engaging conversation that keeps your time enjoyable and entertaining. Plus they look fabulously elegant; making an impressionable.
Independent Escorts pune understands the value of confidentiality and discretion - they will go the extra mile to meet your needs. Simply contact them via text messaging or through their online profiles; they'd be more than delighted to accommodate any request or arrange a romantic date or fun-filled night together.
We provide -
01-may-2024(v.n)
Booking open Available Pune Call Girls Koregaon Park 6297143586 Call Hot Ind...

Booking open Available Pune Call Girls Koregaon Park 6297143586 Call Hot Ind...Call Girls in Nagpur High Profile
Call girls in delhi ✔️✔️🔝 9953056974 🔝✔️✔️Welcome To Vip Escort Services In Delhi [ ]Noida Gurgaon 24/7 Open Sex Escort Services With Happy Ending ServiCe Done By Most Attractive Charming Soft Spoken Bold Beautiful Full Cooperative Independent Escort Girls ServiCe In All-Star Hotel And Home Service In All Over Delhi, Noida, Gurgaon, Faridabad, Ghaziabad, Greater Noida,
• IN CALL AND OUT CALL SERVICE IN DELHI NCR
• 3* 5* 7* HOTELS SERVICE IN DELHI NCR
• 24 HOURS AVAILABLE IN DELHI NCR
• INDIAN, RUSSIAN, PUNJABI, KASHMIRI ESCORTS
• REAL MODELS, COLLEGE GIRLS, HOUSE WIFE, ALSO AVAILABLE
• SHORT TIME AND FULL TIME SERVICE AVAILABLE
• HYGIENIC FULL AC NEAT AND CLEAN ROOMS AVAIL. IN HOTEL 24 HOURS
• DAILY NEW ESCORTS STAFF AVAILABLE
• MINIMUM TO MAXIMUM RANGE AVAILABLE.
Call Girls in Delhi & Independent Escort Service –
CALL GIRLS SERVICE DELHI NCR
Vip call girls in Delhi
Call Girls in Delhi, Call Girl Service 24×7 open
Call Girls in Delhi Best Delhi Escorts in Delhi
Low Rate Call Girls In Saket Delhi
X~CALL GIRLS IN Ramesh Nagar Metro
best Delhi call girls and Delhi escort service.
CALL GIRLS SERVICE IN ALL DELHI …
(Delhi) Call Girls in (Chanakyapuri)
Hot And Sexy Independent Model Escort Service In Delhi Unlimited Enjoy Genuine 100% Profiles And Trusted Door Step Call Girls Feel Free To Call Us Female Service Hot Busty & Sexy Party Girls Available For Complete Enjoyment. We Guarantee Full Satisfaction & In Case Of Any Unhappy Experience, We Would Refund Your Fees, Without Any Questions Asked. Feel Free To Call Us Female Service Provider Hours Opens Thanks.
Delhi Escorts Services 100% secure Services.Incall_OutCall Available and outcall Services provide.
We are available 24*7 for Full Night and short Time Escort Services all over Delhi NCR.
Delhi All Hotel Services available 3* 4* 5* Call Call
Delhi Escorts Services And Delhi Call Girl Agency 100% secure Services in my agency. Incall and outcall Services provide.
We are available 24*7 for Full Night and short Time Escort Services my agency in all over New Delhi
Delhi All Hotel Services available my agency
SERVICES [✓✓✓]
Housewife
College Girl
VIP Escort
Independent Girl
Aunty
Without a Condom sucking )?
Sexy Aunty.DSL (Dick Sucking Lips)?
DT (Dining at the Toes English Spanking)
Doggie (Sex style from no behind)??
OutCall- All Over Delhi Noida Gurgaon 24/7
FOR APPOINTMENT Call/Whatsop / 9953056974Call Girls in Ramesh Nagar Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service

Call Girls in Ramesh Nagar Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service9953056974 Low Rate Call Girls In Saket, Delhi NCR
Kürzlich hochgeladen (20)
result management system report for college project

result management system report for college project
VIP Model Call Girls Kothrud ( Pune ) Call ON 8005736733 Starting From 5K to ...

VIP Model Call Girls Kothrud ( Pune ) Call ON 8005736733 Starting From 5K to ...
Call Now ≽ 9953056974 ≼🔝 Call Girls In New Ashok Nagar ≼🔝 Delhi door step de...

Call Now ≽ 9953056974 ≼🔝 Call Girls In New Ashok Nagar ≼🔝 Delhi door step de...
Coefficient of Thermal Expansion and their Importance.pptx

Coefficient of Thermal Expansion and their Importance.pptx
chapter 5.pptx: drainage and irrigation engineering

chapter 5.pptx: drainage and irrigation engineering
Booking open Available Pune Call Girls Koregaon Park 6297143586 Call Hot Ind...

Booking open Available Pune Call Girls Koregaon Park 6297143586 Call Hot Ind...
Call Girls Walvekar Nagar Call Me 7737669865 Budget Friendly No Advance Booking

Call Girls Walvekar Nagar Call Me 7737669865 Budget Friendly No Advance Booking
Roadmap to Membership of RICS - Pathways and Routes

Roadmap to Membership of RICS - Pathways and Routes
The Most Attractive Pune Call Girls Budhwar Peth 8250192130 Will You Miss Thi...

The Most Attractive Pune Call Girls Budhwar Peth 8250192130 Will You Miss Thi...
Call Girls in Ramesh Nagar Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service

Call Girls in Ramesh Nagar Delhi 💯 Call Us 🔝9953056974 🔝 Escort Service
BSides Seattle 2024 - Stopping Ethan Hunt From Taking Your Data.pptx

BSides Seattle 2024 - Stopping Ethan Hunt From Taking Your Data.pptx
Solid gas system
- 1. Solid-Gas equilibria of CuSO4.5H2O system -Mohamed Minas 20052201017
- 2. There are such system in which two component forms a compound and at a particular temperature it decomposes into another solid and liquid. The composition of liquid state is different here then the solid state. At this point; Compd with Incg MP Original solid + solution These compounds are called as compound with Incongruent melting point. Actually the compound dissociate at this point and the process is called as peritectic reaction. The Incongruent melting point is called as meritectic or peritectic temperature. Incongruent melting point
- 3. Copper sulphate-water vapour system This is a two component system in which one component is solid and other component is water vapour. This phase diagram is drawn between vapour pressure v/s water vapour composition in such a way that by the addition of water vapour to the system at constant temp of 55°C copper sulphate forms three different hydrates at a certain vapour pressure level. The three hydrates are Copper sulphate monohydrate (CuSO4.H2O) Copper sulphate trihydrate(CuSO4.3H20) and Copper sulphate pentahydrate(CuSO4.5H20)
- 5. From Point O to A only Copper sulphate is present so there is an increase in vapour pressure of the system. Point A to B: Now monohydrate starts forming and system remains in equilibrium ; CuSO4 + H20 CuSO4.H20 at 4.5 mm of Hg. From B to C only monohydrate is present so again there is an increase in vapour pressure of the system. Point C to D: Now trihydrate starts forming and system remains in equilibrium ; CuSO4.H20 + 2H20 CuSO4.3H20 at 30.9 mm Hg. From D to E only trihydrate is present so again there is an increase in vapour pressure of the system. Point E to F: Now pentahydrate starts forming and system remains in equilibrium ; CuSO4.3H20 + 2H20 CuSO4.5H20 at 45.5 mm Hg.
- 6. From F to G only pentahydrate is present so only increase in vapour pressure of the system. At point O anhydrous CuSO4, has very low vapour pressure hence no reaction between CuSO4 and water, so by the addition of water vapour the vapour pressure of the system starts increasing. On line OA,BC,DE and FG two phases are present. One is either copper sulphate or its hydrate and other is water vapour so on applying phase rule; F’=C-P+1 ; F=2-2+1=2 i.e. system is monovariant at these lines. On line AB,CD and EF three phases are present. Two solids and one water vapour. There is an equilibrium between these three phases, on applying phase rule; ; F’=C-P+1 ; F=2-3+1=0 i.e. system is invariant at these lines.
- 7. Dehydration of Copper sulphate pentahydrate
- 8. Here the temperature is kept constant and the pressure over the salt is continuously decreased, till the dehydration of the salts and the dissociation equilibrium is set up. From Point A to B only Copper sulphate pentahydrate is present so there is an decrease in vapour pressure of the system. Point A to B: Now pentahydrate starts decomposing and system remains in equilibrium. From B to C only trihydrate is present so again there is an decrease in vapour pressure of the system. Point C to D: Now monohydrate starts forming and system remains in equilibrium. From D to E only monohydrate is present so again there is an decrease in vapour pressure of the system. Point E to F: Now anhydrous CuSO4 starts forming and system remains in equilibrium. The degree of freedom of the curve AB,CD,EF is zero i.e, invarient and degree of freedom of the curve B,D,F is 1 i.e, monovarient.