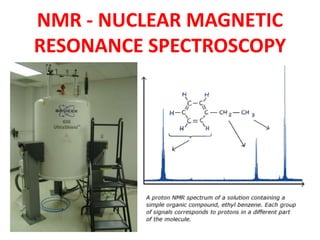
Nmr nuclear magnetic resonance spectroscopy
- 1. NMR - NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
- 2. WHAT WE KNOW… Tool for structure determination. Used for both organic or inorganic compounds..? Easily available..? Expensive or cheap..? Affordable..? Quantitative analysis
- 3. REFERENCES
- 6. INTERNET REFERENCES http://www.youtube.com/watch?v=7aRKAXD4dAg - Type ‘Magritek’ in youtube http://www.youtube.com/watch?v=jRxgX-7FO8g http://www.freelance-teacher.com/videos.htm - Type ‘freelanceteach’ in youtube • http://www.youtube.com/watch?v=uNM801B9Y84
- 8. VECTORS Quantities which have both magnitude and direction. Examples for vectors are Force, velocity, angular momentum, spin etc When two vectors (P and Q) interact with each other, the resultant vector (R) has a different direction and magnitude, which is influenced by the properties of the interacting vectors (P and Q) R = (P2+푸ퟐ + ퟐ푷푸풄풐풔θ)0.5
- 9. SPIN Form of angular momentum. This is an intrinsic property shown by elementary particles and some atomic nuclei. This is a vector quantity, has both magnitude and direction. Particles which posses spin and have charge, interact with magnetic fields, since they generate their own magnetic field
- 10. Bose – Einstein Statistics Fermi – Dirac Stats • Indistinguishable particles • One particle per energy level • Particles have half integral spin • Examples for Fermions are protons, electrons, NO, 2He3 • Indistinguishable particles • Any number of particles can occupy a given energy level • Particles have integral spin. • Examples for Bosons are N2, H2, photons.
- 11. NUCLEAR SPIN Nuclei consist of nucleons, each having spin quantum number of ‘Half’ (1/2) When these nucleons combine to give the nucleus of an atom, the nucleus possesses ‘Spin’ For a nucleus, the number of allowed spin states is quantized and determined by it’s ‘Nuclear Spin Quantum Number’ (Symbol : I) For each nucleus, there are (2I+1) allowed spin states, with integral differences ranging from +I to –I + I, (I-1), (I-2),…., (-I+1), (-I)
- 12. MORNING EXERCISE Exercise: Calculate the allowed spin states for a. Hydrogen (I= ½) b. Chlorine (I= 3/2). You should get 2 spin states for Hydrogen i.e (+1/2 and -1/2) 4 spin states for Cl i.e. (-3/2, -1/2, +1/2, +3/2)
- 13. NMR ACTIVE NUCLEI All nuclei carry charge, they possess spin angular momentum Only nuclei having spin quantum number I > 0 are NMR active. Mass no Atomic no. Spin quantum no. Examples NMR Activity Odd Odd or Even Half Integral ½(H), 3/2 (13C), 5/2 (17O) Active Even Even Zero 0 (12C, 16O) Inactive Even Odd Integral 1(2H, 14N), 3(10B) Active
- 14. OF ENERGY AND POPULATION In the absence of an applied magnetic field, all spin states of a nucleus are degenerate (have equal energy) In a collection of atoms, all spin states should be equally populated. This situation changes, when an external magnetic field is applied. Degeneracy is lost, the spin states have different energies. Also, the population of atoms occupying each state changes.
- 15. ALIGN AND OPPOSE For a Hydrogen nucleus, there are two spin states, (+1/2) and (-1/2) Under an applied field, each nucleus can adopt only one of these states. (+1/2) is of lower energy since it is aligned to the field. This is called α state (-1/2) is of higher energy since it is opposed to the field. This is called the β state
- 16. NUCLEAR MAGNETIC RESONANCE NMR occurs when nuclei aligned to applied field, absorb energy and oppose it. This is a Quantized process , and energy absorbed should be equal to difference in energy between the two spin states. Eabs = E(1/2) – E(-1/2) = hν Energy difference is also a function of applied magnetic field.
- 17. Inaugural Derivation..! Concentrate on the Board..!
- 18. VISUALISE
- 20. COMPARE… Source : Google Images
- 21. SPINNING TOPS AND NUCLEI The nucleus behaves as a spinning top under the influence of an external field. The nucleus undergoes precession around its own axis with an angular frequency ω. Stronger the magnetic field, higher the ω. Precession generates an oscillating electric field, of same frequency If radio waves of same frequency are supplied externally, absorption occurs.
- 22. PROTON FLIPPING Absorption occurs since the electric field generated by the nucleus and the electric field of the incoming radiation undergo coupling. Energy can be transferred from the incoming radiation to the nucleus. Spin change occurs from (+1/2) to (-1/2). This condition is called Resonance.
- 23. ANATOMY Source : Google Images
- 24. LABELLING THE PARTS There are primarily 4 parts : - The Magnet : With a controller to produce a specified magnetic Field - Radio Frequency Source - Detector : To detect the output signal - Recorder : To record the output and plot it against the magnetic field.
- 25. FURTHER DETAILS Sample is taken in a glass tube, placed between 2 poles of a magnet. Sample is exposed to Radio Waves, frequency kept constant. The tube is spun around at a steady rate, such that all parts of the sample get equal exposure to the radiation and the magnetic field. Using electromagnets, the magnetic field strength is varied.
- 26. SOLVENTS As the field strength is varies, the precessional frequency of the proton matches the frequency of incident radiation. Small amount of the compound is taken in 0.5-1 ml of solvent. Solvents used are Deuterated. Common ones are D2O, CDCl3, DMSO. These solvents do not contain protons. Deuterium though being NMR active, does not resonate along with a proton.
- 27. STRUCTURES - Pure CDCl3 and DMSO do not show any peaks in 1H NMR. - However, commercial samples are not 100% pure and hence show peaks. E.g: DMSO – 2.5ppm, CDCl3 – 7.32, D2O – 4.79* Source : Wikipedia Ref: Fulmer, Miller et al : NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist, Organometallics 2010, 29, 2176–2179
- 28. FT NMR The older NMR spectrometers used to excite nuclei of one type at a time. Resonances of individual nuclei are recorded separately and individually. Modern instruments use short (1-10 μs) bursts of energy (radiation) called Pulses. A Pulse contains a range of frequencies, which is enough to excite all the types of protons at the same time.
- 29. Each type of proton, emits radiation of different frequencies. This emission is called a Free Induction Decay. (FID) The intensity of this FID decreases over time as the nuclei lose their excitation. The FID signal is a superimposed combination of all the frequencies emitted. A mathematical method called ‘Fourier Transform’ is used to extract individual frequencies.
- 30. The FT breaks the FID into individual sine or cosine wave components. From these components the individual frequencies can be determined. The computer collects intensity vs time data and converts it into intensity vs frequency data. The final spectrum obtained (I vs ν) is called the FT NMR Spectrum.
- 31. Left : Free Induction Decay Signal Below: The FID Signal broken into a sine wave using FT.
- 33. ADVANTAGES OF FTNMR Entire spectrum recorded, computerized and transformed in few seconds 400 spectra accumulated in approx. 13mins Samples at very low conc. Can be measured. Studies on nuclei with low natural abundance, and small magnetic moments ( 13C, 15N & 17O) Improved spectra for sparingly soluble compounds. Many spectra for same sample : Averaging
- 34. DEFLECTION
- 35. SHIELDING Each proton is in a slightly different chemical environment within the molecule and hence not all protons resonate at the same frequency. Electrons surrounding the protons shield (or protect) the protons from the applied magnetic field. The electrons circulate and form a current. This current generates a secondary magnetic field which opposes the applied field. This is called ‘Diamagnetic Sheilding’
- 36. QUANTITATIVE ASPECTS Greater the electron density around the proton, greater the induced counter field. Proton experiences a lower magnetic field, and hence the precessional frequency of the proton decreases. Due to this the proton undergoes absorption at a lower frequency. Since each proton is in a different environment, each has a different resonance frequency.
- 37. DESHIELDING In some cases, like aromatic nuclei, closed loops of pi-electrons are found. These electrons cause strong diamagnetic shielding around the aromatic nucleus, but the protons align themselves to the magnetic field and hence are deshielded. In other words, when protons align themselves to the direction of the applied field, they are said to be deshielded.
- 38. MEASUREMENTS AND REFERENCES The difference in absorption frequencies is very low (50-100 Hz in field of 60 MHz) The exact resonance frequencies are not easily measurable, and hence a reference compound TMS (Tetramethylsilane) is used. TMS protons are most shielded. TMS gives one sharp peak, which is taken as zero. All other proton resonance frequencies are measured relative to TMS
- 39. NUMERICALS Calculate the resonance frequency of a proton at a.) 1.41 Tesla. b.) 2.35 Tesla. c.) Find the ratio of the given field strengths and the calculated resonance frequencies From this we infer that, for a given proton, the shift in frequency from TMS is field dependent.
- 40. CHEMICAL SHIFT Protons resonate at different frequencies depending on the strength of the applied field and the radiation used. This can be confusing if chemists have spectrometers of different magnetic fields. To avoid this confusion, a new parameter which is independent of the applied field is defined. This parameter is called ‘Chemical Shift’
- 41. MATHEMATICALLY SPEAKING • Values of δ for a given proton are same irrespective of the spectrometer frequency.
- 42. MORE NUMERICALS For example : Bromomethane protons resonate at 162Hz in 60 MHz NMR, but in 100 MHz NMR they resonate at 270 Hz. Calculate δ for both. Calculate the frequency and energy corresponding to 1 ppm shift from TMS in a.) 600 MHz NMR Spectrometer b.) 300 MHz NMR Spectrometer
- 44. SUMMARISING… Chemical shift of frequency. independent Shielding : Opposing the applied magnetic field, peaks at low δ values. E.g : Aliphatic, vinylic, benzylic protons etc Deshielding : Alignment towards applied field. Peaks at higher δ values. E.g : Aromatic protons and protons of aldehydes and carboxylic acids. So tell me where do ketone proton peaks appear..?
- 45. FACTORS AFFECTING CHEMICAL SHIFT Inductive or Electronegativity effects Hybridization effects Hydrogen Bonding Solvent effects Van der Waal’s deshielding Geminal and Vicinal Coupling Proton exchange
- 46. ELECTRONEGATIVITY EFFECTS If electronegative elements are attached to the same carbon of interest, the absorption shifts downfield. More electronegative the atom, more downfield is the absorption. Multiple substituents have a stronger effect Effect decreases over distance This idea can be used to measure electronegativity of elements
- 48. HYBRIDIZATION EFFECTS Higher the s character of the bond, closer the electrons are to the nucleus. Proton resonates downfield. Other effects can affect this order. For sp3 hybridised C atoms 3o > 2o > 1o > strained ring δ Values: 2 1 0
- 49. Sp2 and sp Hybrid Carbons The s character is greater in protons attached to sp2 carbons. Appear at 4.5 – 7 ppm. Aromatic Protons appear at 7-8 ppm, and aldehydic protons at 9-10 ppm. This high shift is caused by another effect called anisotropy. For acetylic hydrogens, anisotropy results in shift at 2-3 ppm, though it has greater s character.
- 50. MAGNETIC ANISOTROPY Protons attached to benzene rings are influenced by 3 magnetic fields. i. External Magnetic field ii. Magnetic field generated by circulating π electrons. Iii. Magnetic field generated by valence electrons around the protons. These fields interact with each other and caused anisotropy. (non uniformity). Benzene protons lie in the deshielded region and hence they appear downfield.
- 51. MAGNETIC ANISOTROPY All groups with π electrons cause anisotropy. In acetylene, the geometry of the generated field is such that the protons get shielded. Hence they appear upfield.
- 54. Hydrogen Bonding In samples that can undergo intermolecular H bonds, the δ value is highly concentration dependent. Intramolecular H bonding is very slightly concentration dependent.
- 55. EXCHANGEABLE PROTONS Protons which can undergo exchange show variable and broad absorptions.
- 56. VARIABLE ABSORPTIONS Acids RCOOH 10.5-12.0 ppm Phenols ArOH 4.0- 7.0 ppm Alcohols ROH 0.5-5.0 Amines RNH2 0.5-5.0 Amides RCONH2 5.0- 8.0 Enols CH=CH-OH > 15
- 57. Functional Group Proton Chemical Shift (ppm) Reason Ethers R-O-CH 3.2-3.8 ppm Electronegativity of oxygens Haloalkanes -CH-I 2.0-4.0 ppm Electronegativity of halogen atom -CH-Br 2.7-4.1 -CH-Cl 3.1-4.1 -CH-F 4.2-4.8 Nitriles -CH-C≡N 2.1-3.0 α- Protons deshielded by C≡N group Amines R-N-H CH-N-Ar- N-H 0.5-4.0 2.2-2.9 3.0-5.0 Protons undergo exchange Resonance removes electron density from nitrogen
- 58. Functional Group Proton Chemical Shift (ppm) Reason Ketones R-CH-C=O 2.1-2.4 Anisoptropy of C=O group Esters 2.1-2.5 3.5-4.8 Anisoptropy of C=O group Electronegativity of Oxygen Carboxylic acids R-COOH -CH-COOH 11.0-12.0 2.1-2.5 Electronegativity of Oxygen Signal usually broad. Exchangeable Amides R(CO)-N-H- -CH-CONH-R( CO)-N-CH- 5.0-9.0 2.1-2.5 2.2-2.9 Variable absorption Deshielded by carbonyl group - Nitrogen’s electronegativity C H2 O O CH2
- 59. INTEGRALS AND INTEGRATION Apart from finding the types of hydrogens present, NMR can also be used to find the relative number of hydrogens. This can be done by calculating the area under each peak. This is done by tracing a vertically rising line over each peak, this line is called the integral. The integral rises by an amount proportional to the area under the peak.
- 62. CALCULATING THE HYDROGENS CONCENTRATION ON THE BOARD..! WORD FILE..!
- 63. ALCOHOLS C-OH – 0.5-5.0 ppm CH-OH – 3.2- 3.8 ppm No spin-spin coupling if exchange is rapid. Exchange promoted by increased temperature, small amounts of acidic impurities and also water content.
- 64. DEUTERIUM SHAKE Used to identify –OH absorption. Add D2O to the sample, and shake. Hydrogen gets replaced by D atom, and peak disappears.
- 65. A compound with molecular formula C8H10 has in its 1HNMR spectrum two signals δ 2.3(6H) and 7.1(4H) deduce the structure p-xylene
- 66. Deduce the structure of an organic compound(C7H8O having following spectral data IR : 3300cm-1( br), 3050cm-1 (m) , 2950cm-1(str) NMR: δ7.1(m,5H) , δ4.4(s,2H) and 3.7(s,1H) C6H5CH2-OH Benzyl alcohol
- 67. Deduce the structure of an organic compound (C8H9NO) having following spectral data IR : 3430cm-1, 1710cm-1 , NMR: δ7.3(m,5H) , δ2.2(s,3H) and 8.3(br,1H) C6H5NH-CO-CH3 acetanilide
- 68. A compound with molecular formula C8H8O shows following NMR peaks Deduce the structure of the compound δ 2.8 (2H, d): δ 7.28 (5H,m) δ 9.78(1H,t) C6H5CH2CH=O Phenyl acetaldehyde
- 69. COUPLING CONSTANT (J) Distance between peaks of a simple multiplets Geminal Coupling 2J : Coupling between 2 protons on same carbon, this carbon can be C-13 .
- 70. COUPLING CONSTANTS OF SOME GROUPS ALKANES: Three types of hydrogens : - Methyl Hydrogens (-CH3): δ 0.7- 1.3 ppm - Methylene hydrogens (-CH2-): δ 1.2 – 1.4 ppm - Methine Hydrogens (-CH--): δ 1.4-1.7 ppm Coupling Constant : 3J = 7-8 Hz
- 71. ALKENES Vinylic hydrogens : (C=C-H) δ 4.5 – 6.5 ppm Allylic Hydrogens : (C=C-C-H) δ 1.6 – 2.6 ppm 3Jtrans = 11 – 18 Hz 3Jcis = 6 -15 Hz 2J = 0-3 Hz 4J = 0-3 Hz
- 72. H H a n g le = 1 0 9 o 2 J = 1 2 -1 8 H z H H a n g le = 1 1 8 o 2J = 5 H z H H a n g le = 1 2 0 o 2J = 0 -3 H z H H C H 3 H 3C C H 3 O a n g le = 1 0 7 o 2 J = 1 7 .5 H z
- 73. Karplus Relationship Relates coupling constant with dihedral angle. variation of Jvic is given by Karplus’s equation 3JH,H = A + B cosф + C cos2ф Modified as : If 0o˂ ф˂90o , Jvic = 8.5cos2 ф-o.28 If 90o˂ ф˂180o , Jvic = 9.5cos2 ф-o.28
- 75. Types of NMR spectrum First order spectra: Spectra shows multiplets obeying n+1 rule and intensities following Pascal’s triangle n+1 rule is applied only if 1) ratio of difference in chemical shift (ΔνHz) of interacting nuclei to their coupling constant J(Hz) is more than 10, ΔνHz/JHz≥10 Values of J of all protons in neighboring groups with protons of interest must be identical
- 76. First Order Spectra: J does not depend on field strength, chemical shift value depends on field strength At field strength ΔνHz/JHz≥10 possible Spectra becomes first order at higher field strength .
- 77. Second Order Spectra If ratio ΔνHz/JHz ˂ 10 l 1H NMR becomes complicated Splitting not obey n+1 rule Intensities of peaks not belong Pascals tringle Also known as non-first order spectrum
- 78. Limitations of the (n+1) rule This is valid only when vicinal inter-proton coupling constants are exactly the same for every successive pair of carbons. H 2 C JAB = JBC = JCD = JDE …. H 2 C CH 2 H 2 C CH 2 A B D C E
- 79. H 16 8 8 4 4 4 4 2 2 4 4 2 2 1 13 3 3 3 1 1 1 4 6 4 1 H H H C C C H H In the above molecule, count the number of 3J H-H couplings, for the 2nd carbon. You will find 4 of them. Hence, 24 = 16 H
- 80. Rules are meant to be broken..? Use 1,1,2-trichlorethane – predict spectrum. This obeys (n+1) rule. Styrene oxide does not… All protons of the side chain appear as quartets. HA HC H Reasons: Coupling constants not equal for vicinal protons. Restricted rotation across the ring. H OH HC
- 81. J1 J2 CH CH2 J1 J2 J2 CH2 O J1 J2 CH2 CH CH J1 J2 J2
- 82. Hindered Rotation N,N- Dimethyl Formamide shows 2 distinct peaks for the methyl protons even though they are chemically equivalent. Free rotation of the methyls around the nitrogen is possible. This is due to resonance, the molecule is forced to adopt a planar geometry making the methyl magnetically non equivalent. At higher temperatures, the rate of rotation increases and a single peak is equivalent.
- 83. Spin System Notations – Pople Notation Each chemically different proton is given a capital letter A, B, C so on… If there are more than one proton of the same type then numerical subscripts are used A2 , B2 etc Protons with widely different chemical shift are denoted with letters far off in the alphabet. E.g AB protons are more closely related than AX or AY protons. If three protons with different chemical shifts exists then notations like AMX, AMY etc are used.
- 84. For protons to give distinct peaks, the ratio of Δν/J (Both measured in Hertz) should be more than 10. For AB protons this ratio is very low compared to that of AX protons. Δν (Hz) is dependent on spectrometer frequency, but J (Hz) is independent of frequency. Hence to get larger Δν/J spectrometer of higher frequencies have to be used. In other words AB protons behave as AX protons in higher frequency spectrometer. Which means that at higher frequencies, second order spectra become first order spectra.
- 86. Some Examples 1,1,2-trichloroethane coupling constant is 7Hz Difference btw CH & CH2 protons is 1.8 δ (5.7-3.9 δ) corresponding to 108Hz (60MHz spectrum) Hence ΔνHz/JHz ˃ 10 If Ha & Hb become more alike relative intensities of peaks do not follow Pascal’s triangle Spectrum deviates from first order During distortion of signals inner signal intensity increases outer signal intensity decreases
