TARGET Factory Audit
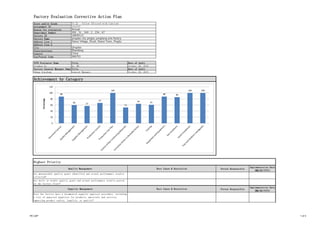
- 1. Factory Evaluation Corrective Action Plan Score and/or Grade Assignment ID Reason for evaluation Department Number Factory ID Factory Name Address Line 1 Address Line 2 City State/province Country Zip/Postal Code TCPS Evaluator Name Title Date of Audit Stephen Yu Sr. MT October 29, 2015 Factory General Manager Name Title Date of Audit Zhang Xiaodong General Manager October 29, 2015 Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Achievement by Category 73.22 - Yellow (Proceed with Caution) 1512737 Annual 200 , 51 , 240 , 2 , 234 , 67 16839113 qingdao city pingdu yongliang arts factory Dianzi Village, Zhudi, Dianzi Town, Pingdu Qingdao Shandong China 266753 Highest Priority Quality Management Root Cause & Resolution Are measureable quality goals identified and actual performance results collected? Are daily or weekly quality goals and actual performance results posted on the factory floor? Supplier Management Root Cause & Resolution Does the factory have a documented supplier approval procedure, including a list of approved suppliers for products, materials and services impacting product safety, legality, or quality? 88 60 57 67 100 52 64 61 88 86 100 100 0 20 40 60 80 100 120 Percentage FE CAP 1 of 3
- 2. Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Does the supplier approval procedure include clear criteria for ongoing assessment and the standards of performance required? Production Control Root Cause & Resolution Are product test requirements and results reviewed prior to production or at the pre-production meeting and is this documented in the meeting notes?Is the identification of raw materials and components adequate to ensure traceability ? Is traceability available from source of raw material and components thru finished product? Are final products suitably marked on packaging and/or product to allow identification and traceability? Are there documented PRODUCTION instructions present at each production operation? Are there documented INLINE INSPECTION instructions at each inspection operation? Does the factory test the traceability system to ensure it works? Control of Non-Conforming Materials Root Cause & Resolution Does the factory have procedures to ensure that customers are notified immediately on issues of product safety or legality (non-compliance with any rule, ban, standard and regulation)? Is there evidence to prove that non-conforming materials and their packaging are handled and disposed of according to customer requirements and/or legal requirements? Are the non-conforming procedures understood by the factory personnel and implemented effectively? Corrective Action or Remedial Action Plans Root Cause & Resolution Is Data collected, such as but not limited to: product testing results, product inspection results, customer complaints and recalls used to initiate corrective action/remedial action? Is there a process to follow-up on each corrective action to ensure execution and prevention of reoccurrence (closed loop)? Training Root Cause & Resolution Has the factory received training on undue influence of third party labs? Is there tailored training for QC, Production, Lab, and Equipment Maintenance? Equipment and Equipment Maintenance Root Cause & Resolution Are procedures in place for actions to be taken if equipment is found not to be operating within specified tolerances and/or limits? Additional Opportunities Document Control Root Cause & Resolution Is the documentation readily accessible by all relevant staff at all times? Supplier Management Root Cause & Resolution Are supplier approval records kept? Does the factory review the performance of suppliers against a defined criteria? FE CAP 2 of 3
- 3. Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Person Responsible Implementation Date (MM/DD/YYYY) Does the factory have documented processes for reviewing product and packaging material in the light box, under the current Target required light source to ensure consistency of color? Production Control Root Cause & Resolution Does the factory use defect samples in the production area to show examples of common defects? Control of Non-Conforming Materials Root Cause & Resolution Does the non-conforming material system allow for operators to identify and flag defective goods on the line? Corrective Action or Remedial Action Plans Root Cause & Resolution Does the factory management periodically review the corrective action (remedial action) system for effectiveness and adoption of preventative actions? Site Conditions Root Cause & Resolution Does the plant have a back up power supply available that will allow production to continue in case of power failure? FE CAP 3 of 3