Periodic table shortened (1)
•Als PPT, PDF herunterladen•
0 gefällt mir•169 views
pd
Melden
Teilen
Melden
Teilen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Andere mochten auch
Andere mochten auch (20)
Ähnlich wie Periodic table shortened (1)
Ähnlich wie Periodic table shortened (1) (20)
PARTS-OF-PERIODIC-TABLEeeeeeetheperiodictrends.pptx

PARTS-OF-PERIODIC-TABLEeeeeeetheperiodictrends.pptx
Chemistry Notes the Periodic Table powerpoint.pptx

Chemistry Notes the Periodic Table powerpoint.pptx
Mehr von Allyse Fritz
Mehr von Allyse Fritz (20)
Kürzlich hochgeladen
Kürzlich hochgeladen (20)
Formation of low mass protostars and their circumstellar disks

Formation of low mass protostars and their circumstellar disks
Call Girls Alandi Call Me 7737669865 Budget Friendly No Advance Booking

Call Girls Alandi Call Me 7737669865 Budget Friendly No Advance Booking
Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b

Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b
Pulmonary drug delivery system M.pharm -2nd sem P'ceutics

Pulmonary drug delivery system M.pharm -2nd sem P'ceutics
Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b

Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b
9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000

9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000
Seismic Method Estimate velocity from seismic data.pptx

Seismic Method Estimate velocity from seismic data.pptx
Connaught Place, Delhi Call girls :8448380779 Model Escorts | 100% verified

Connaught Place, Delhi Call girls :8448380779 Model Escorts | 100% verified
Periodic table shortened (1)
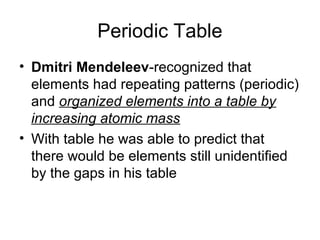
- 1. Periodic Table • Dmitri Mendeleev-recognized that elements had repeating patterns (periodic) and organized elements into a table by increasing atomic mass • With table he was able to predict that there would be elements still unidentified by the gaps in his table
- 2. • Henry Moseley - determined that the number of protons - atomic number (which is unique to each element) would allow the elements to fit into very specific pattern • All elements follow the Periodic Law – chemical and physical properties change periodically with atomic number
- 3. Metals • Most elements are metals • Found to the left of the zigzag line (exception: hydrogen) • Solid at room temp (exception: mercury) Properties: – Shiny – Ductile – Malleable – Good conductors
- 5. Metalloids • Also called semiconductors • Border the zigzag line (exception Al) • Have properties of both metals and nonmetals depending on the conditions • properties: depending on conditions – Brittle – Good conductors – Some shiny (others dull)
- 6. nonmetals • More than half are gases at room temp • To the right of the zigzag line • Properties: – Not malleable or ductile – Not shiny or dull – Poor conductors
- 7. Each square on table • Each square includes: • elements name • chemical symbol (color coded to identify if element is a solid, liquid or gas at room temp) • Atomic number (protons) • Atomic mass (weighed average of isotopes) • Background color (identifies metals, nonmetals and metalloids on table)
- 8. • First letter of chemical symbol is always upper case and any additional letters are lower case • Newest elements have temporary 3 letter symbols • Rows (left to right) are called periods • Rows-determines the number of energy levels – 7 rows on the table • Properties gradually change moving left to right across each row from reactive (group 1) to non- reactive (group 18)
- 9. Energy Levels • 1st energy level – 2 valence electrons (max) • 2nd energy level – 8 valence electrons (max) • 3rd energy level – 18 valence electrons (max) • And so on…. • Each energy level can have less valence electrons but they can not have more than the maximum valence electrons.
- 10. • Columns (up and down) are called groups or family • Elements in the same group or family have similar properties moving up and down each column • Each element in a family has the same number of valence electrons in the last energy level • Group number determine the valence electrons (ex: group one – all elements in group 1 have 1 valence electron, all of the elements in group 2 have 2 valence and so on)
- 11. Bonds • To form bonds, elements must reach a full state of 8 valence electrons in the last energy level (octet rule) • (Exception: Helium - would be first energy level which is full at 2)
- 12. Group 1: Alkali metals • Metals • Valence 1 • Very reactive with H2O, O2 and other elements • Makes compounds with halogens (group 17) easily to form salts
- 13. Group 2 – Alkaline-Earth Metals • Metals • Valence 2
- 14. Group 3 – 12: Transition • Metals • Valence 1 or 2 (depending on element)
- 15. Lanthanides and Actinides (Rare earth metals) • In periods 6 and 7 and appear at the bottom of the periodic table to keep table from being to wide • Lanthanides are shiny reactive metals • Actinides are unstable radioactive • All elements after Pu-94 (plutonium) are man-made in labs and don’t occur in nature
- 16. Group 13: Boron Group • Has 1 metalloid and 4 metals • Valence 3
- 17. Group 14-Carbon group • 1 nonmetal, 2 metalloids and 2 metal • Valence 4 • Carbon forms organic compounds (all living things contain carbon), makes more compounds than any other element
- 18. Group 15-Nitrogen Group • 2 nonmetals, 2 metalloids, 1 metal • Valence 5 • P is extremely reactive and only appears in compounds
- 19. Group 16-Oxygen Group • 3 nonmetals, 1 metalloid, and 1 metal • Valence 6
- 20. Group 17-Halogens • Nonmetals • Valence 7 • has violent reactions with alkali-metals (group 1) to form salt compounds – Highly reactive with other elements – Do not appear in nature alone only in compounds
- 21. Group 18-Noble Gases • Nonmetals • Valence 8 valence (full level) • except helium which has 2 valence electrons, which makes helium full • very un-reactive – inert • Do not form compounds
- 22. Hydrogen • Nonmetal • Valence 1 electron in last energy level so it is set above the alkali metals • Properties: even though above metal category, has properties of nonmetals • Most abundant element in universe, makes up stars
- 23. • Protons = Atomic number • Electrons = protons • Neutrons = mass number (rounded) minus the protons • Protons do not change in a atom, • neutrons can change (isotopes), • electrons can be shared or transferred (when bonds are made)
- 24. Reflection Questions • 1. What are the seven horizontal rows of the table called? How are the elements of a row related? • 2. What are the vertical columns of the table called? How are the elements of each column related? 3.What basic information is contained in the table?