Diborane
- 2. Methods Of Preparetion It is the simplest boron hydride. It is prepared by treating boron Trifluoride with LiAlH4 in diethyl ether 4BF3 + 3LiAlH4 2B2H6 +3LiF + 3ALF3 In a laboratory the diborane is prepared by the oxidation of sodium borobhydride with iodine 2NaBH4 + I2 B2H6 +2NaI +H2 In industry it is prepared by the reaction of BF3 with sodium hydride 2 BF3 + 6 NaH 450 K B2H6 + 6 NaF
- 3. PRORPERTIES 1) Diborane is a colorless, highly toxic gas. It has boiling point ok 180 K 1) Diborane catches fire spontaneously when it is exposed to atmospheric air it burns with oxygen. The reaction is exothermic releasing a lager amount of energy B2H6 + 3O2 B2O3 + 3H2O ΔH= - 1976 K J mol-1 3) it is readily hydrolyzed by water to form boric acid B2H6 + 6H2O 2B(OH)3 + 6H2
- 4. 4) With Lewis bases, diborane first undergoes cleavage (breaking) to form borane (BH3) which then reacts to form adducts B2H6 + 2NMe 2BH3 . NMe B2H6 + 2CO 2BH3 . CO 5) with ammonia an addition product B2H6.NH3formulated as [ BH2(NH3)2]+ [BH4]- is formed which then decomposes on heating at 473K to give a volatile compound called borazole (Borazine) 3 B2H6 + 6NH3 [ BH2(NH3)2]+ [BH4]- 473K 2B3N3H6 + 12H2 ( borazole)
- 5. BORAZOLE (BORAZINE) This compound is called inorganic benzene in view of its ring structure with alternate NH and BH groups. Borazole contains 3 double bonds and has 6 electron system which is similar to benzene
- 6. 6)Many metal hydrides react with diborane to form tetrahydridoborate which contain [BH4]- tetrahedral ion. 2MH + B2H6 2M+ [BH4]- Where (M=Li or Na), for ex: 2LiH + B2H6 2Li+ [BH4]-
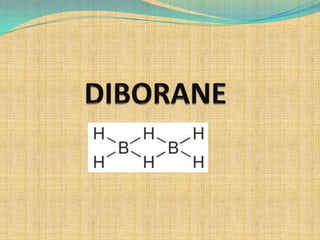
- 7. STRUCTURE OF DIBORANE X-ray diffraction has shown the following structure of diborane Here B atoms undergo sp3 hybridization. There are four terminals B-Ht normal covalent bonds which are quite strong and two bridge B……Hb…..B which are different from normal covalent bonds and are quite weak The four Ht molecules and b atoms lie in the same plane while one Hb lies above and one below the plane
- 8. As there are 12 electrons and 8 bonds in B2H6 molecule it is an electron deficient molecule Because of their resemblance to a banana these are also called banana bonds shown in the structure. Hence diborane molecule has two electrons less than the maximum required number thus it is an electron deficient molecule
- 9. USES OF DIBORANE Diborane has been suggested as a rocket propellant and experimentally fired but not used in any in service rocket as a rubbervulcaniser as a catalyst for hydrocarbon polymerization as a flame-speed accelerator, and as a doping agent for the production of semiconductors. It is also an intermediate in the production of highly pure boron for semiconductor production. It is also used to coat the walls of tokomaks to reduce the amount of heavy metal impurities in the plasma.
- 10. The End
