Subatomic particles and atomic structure
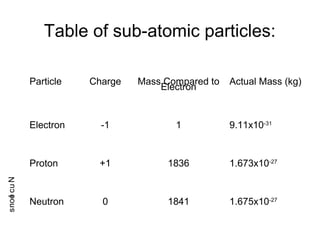
- 1. Table of sub-atomic particles: Particle Charge Mass Compared to Electron Actual Mass (kg) Electron -1 1 9.11x10-31 Proton +1 1836 1.673x10-27 Neutron 0 1841 1.675x10-27 Nucleons
- 2. Definitions: • atomic number: Number of protons in the Nucleus • mass number: Number of protons + number of neutrons • atomic mass: mean mass of all isotopes (measured in AMU – Atomic Mass Units) Atomic number and mass number are counts. Atomic mass has units of mass (AMU).
- 3. Isotopes • For a particular type of atom (say, Iron) you must have exactly 26 protons and 26 electrons. • The number of neutrons may vary, resulting in isotopes. Example: Hydrogen Deuterium
- 4. What holds an atom together? • The electric charge of the proton and electron hold an atom together. Gravity doesn’t have much sway at this size – the electric force is much stronger here. What is the rule for North and South poles of magnets? Positive and negative charges are similar: opposites attract, likes repel.
- 5. Like Charges Repel • Given that like charges repel, why do we have solids? – Electrons move around, temporary polarization, sharing of electrons between two nuclei • Okay, so they can attract. Why don't the just form a blob? – The nuclei don't like each other.
- 6. How close can atoms get? -0.5 0 0.5 1 1.5 0 1 2 3 Force(relative) Distance (relative) Force between two atoms Above zero = repulsive force Below zero = attractive force There is a limit to how close two atoms can be due to the negatively charged electron clouds.
- 7. Do we ever touch? • What happens when two negative objects get close together? • The electron clouds are negative, so what happens when two atoms get close? Either: – They repel each other – They react chemically We do not actually ‘touch’ objects in the way we usually think we do.
- 8. Imagining a New Force • One billions and billions of times stronger than gravity. • One that, like gravity, loses strength as the square of the distance. • One that unlike gravity can be either attractive OR repulsive. This is the electric force.
- 9. There are two types of particles that interact in the electric force: P (positive) and N (negative) Opposites attract, likes repel. P N P Repulsive Attractive N Attractive Repulsive
- 10. The Electric Force Finally, Suppose we have equal numbers of these two types of particles (P and N). Well, we do. P is positively charged protons, N is negatively charged electrons. They clump together into atoms, which have an overall neutral charge – thus preventing a big rip or a big crush.
- 11. Coulomb’s Law 2 21 ** d qqk F = • Force is proportional to charge. • Charge is measured in Coulombs. 1C = 6.25*1018 electrons of charge. • Force is inversely proportional to the square of distance. • k is a constant value (think of it as being ‘like pi’ but not 3.14). q1 q2 d
- 12. Inverse Square Law Author: Borb, GNU Free Documentation License As distance from source increases, the area of a shell around the source increases as the square of distance. So if the number of ‘lines of force’ are constant, the density will decrease as the square of distance.
- 13. • Outer most electrons are weakly held. • These same outer electrons are responsible for most of a substance’s chemical properties. • Some substances hold electrons more weakly than others (DEMO – hair vs. plastic).
- 14. Conservation of Charge • In the processes you witness today no electrons or protons are created or destroyed. • Just as energy is conserved, so is charge conserved – the universe’s net charge is a constant. • There are no known violations of this principle (it’s more than a theory, we consider it a law).
- 15. Polarization • Electrons are very light (about 1/2000th the mass of a proton or neutron). • They can easily be pushed around by the electric force. • Imagine the electron cloud getting displaced slightly from the nucleus at the center...
- 16. Demo: Induced Polarization • demo balloon on wall: The wall is not charged, but the balloon sticks – electrons in wall get pushed around.
- 17. Example Problem • When you rub a balloon on your hair, does it become charged? • Does your hair become charged? • When you then stick the balloon to a wall (assuming it is dry enough to work) is the WALL charged?
- 18. Inherent Polarization: Water • Water is a polar molecule • The oxygen carries a partial negative charge and the hydrogens carry a partial positive charge. • Oxygen has a stronger ‘hold’ on electrons. • Water can ‘hydrogen bond’ through these weak partial charges, which makes water unusually stable...and allows us to have some fun. Image courtesy Qwerter, GNU Free Documentation License
- 19. Demo: Water and an electric force • Water is a dipole. – dipole means there is a slight charge separation. • Water, since it is charged, will interact with another charged object. + - - - -
- 20. Atoms: attraction and repulsion • Repulsion close -electrons in each atom push against each other • No force far away – atoms are overall neutral • What of the attraction region? Polarization at work. -0.5 0 0.5 1 1.5 0 1 2 3 Force(relative) Distance (relative) Force between two atoms
- 21. Example Problem • Is a polarized object charged?
- 22. Electric Fields An Electric Field is similar to a gravitational field (we live in Earth’s gravitational field). It is also similar to a magnetic field (you can see magnetic field lines by pouring iron filings on a magnet). • A charged particle in an Electric Field will experience a force.
- 23. Microwaves – How they Work • Water is polar. • Microwaves are electromagnetic fields. • The frequency of a microwave oven is near a resonant frequency of rotation for the water. • The water keeps getting banged back and forth. • Motion = heating. Things near the water get hit by the water and are heated.
- 24. Microwaves: the picture Electric field Direction in electromagnetic wave Time Water molecule Orientation
- 25. Conductors • Electrons in conductors are very mobile. • Will always separate so as to cancel the electric field inside. • Faraday Cage: using of metal to create a structure that shields against electric fields. No Electric Field Inside - - - - + + + + + + - - - +
- 26. Van de Graff – Demo (it was broken last time I tried to find it so if it has been fixed you will see it else, sorry!) Schematic view of a classical Van De Graaf generator. 1. hollow metallic sphere (with positive charges) 2. electrode connected to the sphere, a brush ensures contact between the electrode and the belt 3. upper roller (for example in plexiglass) 4. side of the belt with positive charges 5. opposite side of the belt with negative charges 6. lower roller (metal) 7. lower electrode (ground) 8. spherical device with negative charges, used to discharge the main sphere 9. spark produced by the difference of potentials Image and text by Dake, Made available under Creative Commons Attribution ShareAlike 2.5
- 27. The Periodic Table and Chemical Bonding
- 29. Metals • Highly conductive of heat and electricity. • Ductile (may be pulled into wires) • Malleable (may be pounded flat)
- 30. Nonmetals • Poor conductors of both heat and electricity. • Solids are brittle – not malleable or ductile. • Many nonmetals are gasses at room temperature.
- 31. Metalloids • Properties in between metals and nonmetals. • Semiconductors – basis of modern civilization. (May be more like a metal or more like a nonmetal depending on position – closer to metals, metallic and vice-versa)
- 32. Semiconductors in action • Works like a garden hose: squeeze down (G to right hand side), decrease flow of electrons from Souce (S) to Drain (D). • Switching! On a tiny scale. 731 million of these in a chip ½” on a side (Intel’s latest chips).
- 33. A brief Interlude: why is the periodic table structured the way it is? • The periodic table is a map of electronic structure. • First two columns represent filling simplest “orbital” – 2 electrons may fit (except He is displaced) • Last six columns represent filling of next simplest “orbital” – 6 electrons will fit. • Similar for the inner 10 and the odd two rows of 14 displaced to the bottom.
- 34. Giving names to some parts of the periodic table:
- 35. A look across the table: periods Some properties change in a regular way as you go across a row (natural enough, as the valence “shell” is filling up as you go). • Size decreases • Electronegativity (how much the atom wants an electron) goes up.
- 36. Columns: Grouping up All elements in the same column have the same valence (outer) electron arrangement. • Size goes up as you go down a column • Electronegativity goes down • Elements in the same column tend to have similar properties For example: Cu, Ag, Au all in one group – and are among the few elements to be found naturally in pure forms; they are unusually nonreactive)
- 37. Size
