China medical device approval chart - EMERGO
•
2 likes•2,897 views
Easy to understand chart describes the CFDA medical device registration process in China.
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Europe CE Marking for medical devices under new MDR

Europe CE Marking for medical devices under new MDR
South Korea medical device approval chart - Emergo 

South Korea medical device approval chart - Emergo
Regulatory approval process for invitro diagnostics in us

Regulatory approval process for invitro diagnostics in us
The European Medical Device Regulations - analysis of the final text

The European Medical Device Regulations - analysis of the final text
Comparison of Clinical Trial Application requirement of India, USA and Europe.

Comparison of Clinical Trial Application requirement of India, USA and Europe.
Europe IVD medical registration and approval chart - EMERGO

Europe IVD medical registration and approval chart - EMERGO
Viewers also liked
Academic Report on Singapore HSA Class D and Australia TGA Class III Medical ...

Academic Report on Singapore HSA Class D and Australia TGA Class III Medical ...Asia Medical Supplies
Viewers also liked (12)
Chinese Food and Drug Administration (CFDA) Regulatory Approval Process: Medi...

Chinese Food and Drug Administration (CFDA) Regulatory Approval Process: Medi...
Japan PMDA Medical Device Regulatory Approval Process

Japan PMDA Medical Device Regulatory Approval Process
Academic Report on Singapore HSA Class D and Australia TGA Class III Medical ...

Academic Report on Singapore HSA Class D and Australia TGA Class III Medical ...
Sponsor Information and Training day Session C2 - IVDs: Application audits (t...

Sponsor Information and Training day Session C2 - IVDs: Application audits (t...
Regulation of IVD medical devices - presentation at National Histotechnology ...

Regulation of IVD medical devices - presentation at National Histotechnology ...
Summary Report / R&D Evaluation Methodology and Funding Principles

Summary Report / R&D Evaluation Methodology and Funding Principles
Similar to China medical device approval chart - EMERGO
Similar to China medical device approval chart - EMERGO (20)
Strategies for Device Approval in China, India, South Korea and Australia

Strategies for Device Approval in China, India, South Korea and Australia
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
Registration of medical devices with Brazil's Anvisa 

Registration of medical devices with Brazil's Anvisa
Regulation Governing Clinical Trials In India,USA and Europe. 

Regulation Governing Clinical Trials In India,USA and Europe.
rules, regulation and guideline for medical devices

rules, regulation and guideline for medical devices
Recently uploaded
(Deeksha) 💓 9920725232 💓High Profile Call Girls Navi Mumbai You Can Get The Service Of A Mumbai Call Girl At Any Time
WHATSAPP On Here:9920725232
Today call girl service available 24X7*▬█⓿▀█▀ 𝐈𝐍𝐃𝐄𝐏𝐄𝐍𝐃𝐄𝐍𝐓 CALL 𝐆𝐈𝐑𝐋 𝐕𝐈𝐏 𝐄𝐒𝐂𝐎𝐑𝐓 SERVICE ✅
⭐➡️HOT & SEXY MODELS // COLLEGE GIRLS
AVAILABLE FOR COMPLETE ENJOYMENT WITH HIGH PROFILE INDIAN MODEL AVAILABLE HOTEL & HOME
★ SAFE AND SECURE HIGH CLASS SERVICE AFFORDABLE RATE
★ 100% SATISFACTION,UNLIMITED ENJOYMENT.
★ >> 03-05-2024 (GRV)
★ All Meetings are confidential and no information is provided to any one at any cost.
★ EXCLUSIVE PROFILes Are Safe and Consensual with Most Limits Respected
★ Service Available In: - HOME & HOTEL 24x7 :: 3 * 5 *7 *Star Hotel Service .In Call & Out call SeRvIcEs :
★ A-Level (5 star escort)
★ Strip-tease
★ BBBJ (Bareback Blowjob)Receive advanced sexual techniques in different mode make their life more pleasurable.
★ Spending time in hotel rooms
★ BJ (Blowjob Without a Condom)
★ Completion (Oral to completion)
★ Covered (Covered blowjob Without condom
100% SAFE AND SECURE 24 HOURS SERVICE AVAILABLE HOME AND HOTEL SERVICES(Deeksha) 💓 9920725232 💓High Profile Call Girls Navi Mumbai You Can Get The S...

(Deeksha) 💓 9920725232 💓High Profile Call Girls Navi Mumbai You Can Get The S...Ahmedabad Call Girls
Hello, Guys welcome to Manalifun Goa Escort service. Are you want Top call girls in Goa at just ₹10000 then no further anywhere because we have a large number of local beautiful girls. We are a genuine platform to provide unlimited classification escort ads service without any commission. 9316020077
Here many Goa Independent call girls and ladies, publish their ads. Our call girl in Goa is well-known for real sexual fun in Goa. We are not allow any prostitute to work here without checking the details, Firstly all ads check by our team then we publish them here. So don’t hesitate to book Low rate call girls in Goa. 9316020077
Goa call girls: A real wonder in Goa
Who are the best Goa Escort Service provider for Goa call girls
High-Class call girls in Goa escort service for 100% Satisfaction
Choose a trusted call girl service in Goa with Us +91-9316020077
Goa Escorts Provide 100% Client Satisfaction
How Our Goa Call Girls Are Perfect For Instant Satisfaction
100% Guaranteed Goa call girls will make you excited
How to Find Cheap Call Girls in Goa
Our Reliable Escort Service in Goa Local Areas
Goa Escorts (cheap escort service in Goa)
Rate Chart of Goa call girls, (call girl Rate in Goa)
5-star hotel For Goa call girls service
Call girls in Goa are the ideal sex partner for you
BOOK YOUR FAVORITE Goa CALL GIRLS SERVICE WITH US CALL! US NOW~ 9316020077
Best way to Hire call girls in Goa
What’s the cost of escort service in Goa
North Goa Call Girls
Location :-
Baga , Caclangute , Candolim , Anjuna , Panaji Arpora , Vagator , Morjim , Siolim , Mandrem , Arambol , etc.
Vasco , Bambolim , Madgaon, Colva , Etc9316020077📞Goa Call Girls Numbers, Call Girls Whatsapp Numbers Goa

9316020077📞Goa Call Girls Numbers, Call Girls Whatsapp Numbers Goarussian goa call girl and escorts service
Recently uploaded (20)
Hubli Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

Hubli Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
Bhagalpur Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

Bhagalpur Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
nagpur Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

nagpur Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
(Deeksha) 💓 9920725232 💓High Profile Call Girls Navi Mumbai You Can Get The S...

(Deeksha) 💓 9920725232 💓High Profile Call Girls Navi Mumbai You Can Get The S...
coimbatore Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

coimbatore Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
Tirupati Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

Tirupati Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
Call Girl Gorakhpur * 8250192130 Service starts from just ₹9999 ✅

Call Girl Gorakhpur * 8250192130 Service starts from just ₹9999 ✅
Call Girls Service Anantapur 📲 6297143586 Book Now VIP Call Girls in Anantapur

Call Girls Service Anantapur 📲 6297143586 Book Now VIP Call Girls in Anantapur
Jaipur Call Girls 9257276172 Call Girl in Jaipur Rajasthan

Jaipur Call Girls 9257276172 Call Girl in Jaipur Rajasthan
bhubaneswar Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

bhubaneswar Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
Mangalore Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet

Mangalore Call Girls 👙 6297143586 👙 Genuine WhatsApp Number for Real Meet
Call Girls Hyderabad Just Call 9907093804 Top Class Call Girl Service Available

Call Girls Hyderabad Just Call 9907093804 Top Class Call Girl Service Available
Call Girls Patiala Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Patiala Just Call 8250077686 Top Class Call Girl Service Available
VIP Call Girls Noida Jhanvi 9711199171 Best VIP Call Girls Near Me

VIP Call Girls Noida Jhanvi 9711199171 Best VIP Call Girls Near Me
Best Lahore Escorts 😮💨03250114445 || VIP escorts in Lahore

Best Lahore Escorts 😮💨03250114445 || VIP escorts in Lahore
Escorts Service Ahmedabad🌹6367187148 🌹 No Need For Advance Payments

Escorts Service Ahmedabad🌹6367187148 🌹 No Need For Advance Payments
Kochi call girls Mallu escort girls available 7877702510

Kochi call girls Mallu escort girls available 7877702510
💚 Punjabi Call Girls In Chandigarh 💯Lucky 🔝8868886958🔝Call Girl In Chandigarh

💚 Punjabi Call Girls In Chandigarh 💯Lucky 🔝8868886958🔝Call Girl In Chandigarh
9316020077📞Goa Call Girls Numbers, Call Girls Whatsapp Numbers Goa

9316020077📞Goa Call Girls Numbers, Call Girls Whatsapp Numbers Goa
China medical device approval chart - EMERGO
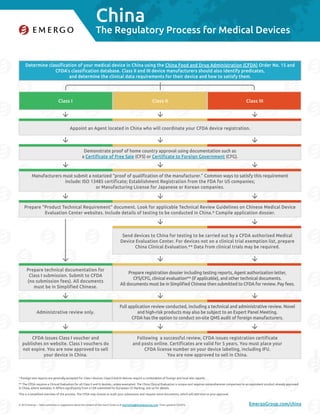
- 1. China © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 12/2016. EmergoGroup.com/china Determine classification of your medical device in China using the China Food and Drug Administration (CFDA) Order No. 15 and CFDA’s classification database. Class II and III device manufacturers should also identify predicates, and determine the clinical data requirements for their device and how to satisfy them. Appoint an Agent located in China who will coordinate your CFDA device registration. Demonstrate proof of home country approval using documentation such as a Certificate of Free Sale (CFS) or Certificate to Foreign Government (CFG). Manufacturers must submit a notarized “proof of qualification of the manufacturer.” Common ways to satisfy this requirement include: ISO 13485 certificate; Establishment Registration from the FDA for US companies; or Manufacturing License for Japanese or Korean companies. Prepare “Product Technical Requirement” document. Look for applicable Technical Review Guidelines on Chinese Medical Device Evaluation Center websites. Include details of testing to be conducted in China.* Compile application dossier. Prepare technical documentation for Class I submission. Submit to CFDA (no submission fees). All documents must be in Simplified Chinese. Administrative review only. CFDA issues Class I voucher and publishes on website. Class I vouchers do not expire. You are now approved to sell your device in China. Send devices to China for testing to be carried out by a CFDA authorized Medical Device Evaluation Center. For devices not on a clinical trial exemption list, prepare China Clinical Evaluation.** Data from clinical trials may be required. Prepare registration dossier including testing reports, Agent authorization letter, CFS/CFG, clinical evaluation** (if applicable), and other technical documents. All documents must be in Simplified Chinese then submitted to CFDA for review. Pay fees. Full application review conducted, including a technical and administrative review. Novel and high-risk products may also be subject to an Expert Panel Meeting. CFDA has the option to conduct on-site QMS audit of foreign manufacturers. Following a successful review, CFDA issues registration certificate and posts online. Certificates are valid for 5 years. You must place your CFDA license number on your device labeling, including IFU. You are now approved to sell in China. Class I Class II Class III 5001-1216 The Regulatory Process for Medical Devices * Foreign test reports are generally accepted for Class I devices; Class II and III devices require a combination of foreign and local test reports. ** The CFDA requires a Clinical Evaluation for all Class II and III devices, unless exempted. The China Clinical Evaluation is unique and requires comprehensive comparison to an equivalent product already approved in China, where available. It differs significantly from a CER submitted for European CE Marking. Ask us for details. This is a simplified overview of the process. The CFDA may choose to audit your submission and request more documents, which will add time to your approval.
- 2. China Notes 1. The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Devices which are novel in the Chinese market may require an Expert Panel Meeting, which will add 3-6 months to the review process. The CFDA also reserves the right to audit the manufacturing facility. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. 2. Registrations remain valid for the time specified as long as you do not make changes to the information on the CFDA certificate, its attachment, or the technical requirement. 3. We recommend starting the re-registration process no later than the time period specified above. However, please consult with your distributor or regulatory expert well before this suggested time to avoid any lapse in your registration. 4. Prices in US Dollars for a single device. 1 = Less than $5,000; 2 = $10,000 - $15,000; 3 = $15,000 - $30,000; 4 = $30,000 - $50,000; 5 = $50,000 or more. Estimated cost includes registration application fees, in-country representation, submission preparation consulting, and translation of documents, if required. Costs assume you already have approval for your device in the United States, Europe, Canada, Australia or Japan. Costs do NOT include product testing, clinical trials or QMS implementation, if applicable. EmergoGroup.com/china Device classification in China Class I Class II Class III How long you should expect to wait after submission until approval is granted1 < 1 week 12-20 months* 12-22 months* Validity period for device registrations 2 Does not expire 5 years 5 years Registration renewal should be started this far in advance3 Not applicable 18-24 months 18-24 months Complexity of the registration process for this classification4 Overall cost of gaining regulatory approval5 5001-1216 Low High Simple Complex Low High Simple Complex Low High Simple Complex © 2016 Emergo – Have comments or suggestions about the content of this table? Email us at marketing@emergogroup.com. Table updated 12/2016. Time, Cost, and Complexity of Registration * These review times presume the CFDA requests additional information and/or additional testing. Most manufacturers will receive such a requst. This is a simplified overview of the process. The CFDA may choose to audit your submission and request more documents, which will add time to your approval. 5 5 5 5 2 2
