short material kinetics.pdf
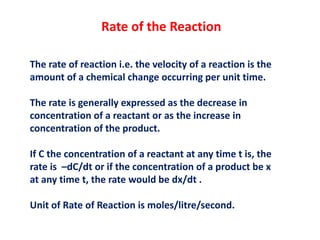
- 1. Rate of the Reaction The rate of reaction i.e. the velocity of a reaction is the amount of a chemical change occurring per unit time. The rate is generally expressed as the decrease in concentration of a reactant or as the increase in concentration of the product. If C the concentration of a reactant at any time t is, the rate is –dC/dt or if the concentration of a product be x at any time t, the rate would be dx/dt . Unit of Rate of Reaction is moles/litre/second.
- 2. Factors influencing the rate of reaction (i) Temperature – For an Increase of temp to 100o rate may doubled or trippled (ii) Concentration of the reactants : Number of reacting molecules undergo effective collision may increase and rate enhances (iii)Nature of reactants : Nature of bonds in reacting molecules. Less bond rearrangement faster the reaction. (iv) Catalysts enhances rate by descreasing the activation energy (v) Radiation – Rate of photochemical reactions enhanced by light absorption
- 3. Order of the Reaction
- 4. Order of the Reaction
- 5. Molecularity of a Reaction
- 6. Differences between Order and Molecularity For Elementary Reactions
- 10. Or
- 12. Examples of First Order Reactions
- 16. Units of Rate Constant
- 17. Units of Rate Constant
- 19. The rate law expression for zero order reaction is, [A]t= −kt[A]0 Where [A]0 and [A]t are the concentration of the reactant at time t =0 and after time t respectively. Half Life Period of a Zero Order Reaction
- 20. Half Life Period of a First Order Reaction
- 21. Half Life Period of a Second Order Reaction
- 24. Theory of Reaction Rates
- 33. Transition State Theory or Activated Complex Theory or Theory of Absolute Reaction Rates
- 34. PE diagram for Exothermic Reaction PE diagram for Endothermic Reaction
- 42. Classifications
- 51. The steady-state approximation is a method used to derive a rate law. The method is based on the assumption that one intermediate in the reaction mechanism is consumed as quickly as it is generated. Its concentration remains the same in a duration of the reaction. Thus, the system has reached a steady-state. In cases where the reactants are investigated under such conditions that the slowest rate-determining step does not exist, one assumes the steady-state approximation, for the transient, or short-lived, intermediate species. Reactants I1 → I2 → I3……..In The rate of formation of an intermediate is equal to the rate of its decomposition so that Steady state approximation is used to check the consistency of the rate law. Steady State Approximation
- 52. Chain Reactions The main types of steps in chain reaction are of the following types. 1. Initiation (formation of active particles) 2. Propagation (may comprise several elementary steps in a cycle, where the active particle through reaction forms another active particle which continues the reaction chain by entering the next elementary step). 3. Termination with product formation A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. Eg: The pyrolysis (thermal decomposition) of acetaldehyde CH3CHO (g) → CH4 (g) + CO (g)
- 53. Chain initiation: The chain is initiated (started) by UV light breaking a chlorine molecule into free radicals. Cl2 → 2Cl. Chain propagation reactions : These are the reactions which keep the chain going. CH4 + Cl. → .CH3 + HCl .CH3 + Cl2→ CH3Cl + Cl. Chain termination reactions: These are reactions which remove free radicals from the system without replacing them by new ones. 2Cl. → Cl2 .CH3 + Cl. → CH3C l .CH3 + .CH3→ CH3 CH3 Photochemical Chlorination of methane CH4 + Cl2 → CH3Cl + HCl
- 54. The reaction H2 + Br2 → 2 HBr proceeds by the following mechanism:[ Initiation Br2 + hν → 2 Br• (photochemical) each Br atom is a free radical, representing an unpaired electron. Propagation (here a cycle of two steps) Br• + H2 → HBr + H• H• + Br2 → HBr + Br• (The sum of these two steps corresponds to the overall reaction H2 + Br2 → 2 HBr, with catalysis by Br• Termination H. + Br. → HBr 2 Br• → Br2 Chain Reaction : Hydrogen Bromine Reaction
