Chemical Equilibrium and Equilibrium Constants
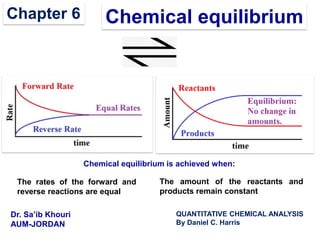
- 1. Chemical equilibriumChapter 6 The rates of the forward and reverse reactions are equal Chemical equilibrium is achieved when: The amount of the reactants and products remain constant Dr. Sa’ib Khouri AUM-JORDAN QUANTITATIVE CHEMICAL ANALYSIS By Daniel C. Harris
- 2. 2 Chemical Kinetics and Chemical Equilibrium A + B C + D kf kr Forward Rate = kf [A] [B] Reverse Rate = kr [C] [D] At Equilibrium: Forward Rate = Reverse Rate kf [A] [B] = kr [C] [D] kf kr [C] [D] [A] [B] = K =
- 3. This chapter introduces equilibriums for the solubility of ionic compounds, complex formation, and acid-base reactions. The symbol [ ] stands for the concentration at equilibrium state Capital letter stands for a chemical species, and small letter denotes stoichiometry coefficient K >> 1 Favor products K << 1 Favor reactants
- 4. 1. Concentrations of solutes should be expressed as moles per liter. Homogeneous equilibrium applies to reactions in which all reacting species are in the same phase. N2O4 (g) 2NO2 (g) e.g. 2. Concentrations of gases should be expressed in bars. 3. Concentrations of pure solids, pure liquids, and solvents are omitted because they are unity. Practically, no units for the equilibrium constant Heterogeneous equilibrium applies to reactions in which reactants and products are in different phases. CaCO3 (s) CaO (s) + CO2 (g) e.g. K = PNO2 2 PN2O4 K = PCO2
- 5. 5 Kp = 2 PNO PO 2 PNO 2 2 PO2 = Kp PNO 2 2 PNO 2 = 347 atm Example The equilibrium constant Kp for the reaction is 158 at 1000K. What is the equilibrium pressure of O2 if the PNO2 = 0.400 atm and PNO = 0.270 atm? 2NO2 (g) 2NO (g) + O2 (g) Solution PO2 = 158 x (0.400)2 (0.270)2
- 6. Equilibrium constant for reverse reaction Equilibrium constant for forward reaction Manipulating Equilibrium Constants When the equation for a reversible reaction is written in the opposite direction, the equilibrium constant becomes the reciprocal of the original equilibrium constant.
- 7. If n reactions are added, the overall equilibrium constant is the product of n individual equilibrium constants. If two reactions are added, the new K is the product of the two individual values: Equilibrium constant for sum of reactions:
- 8. The third reaction can be obtained by reversing the second reaction and adding it to the first reaction: Solution
- 9. Enthalpy, Entropy, and Free Energy Enthalpy change, ΔH: the heat absorbed or released when the reaction takes place. The standard enthalpy change, ΔHº : the heat absorbed or released when all reactants and products are in their standard states. Endothermic reaction: positive value of ΔH Exothermic reaction: negative value of ΔH e.g. Equilibrium and Thermodynamics Equilibrium is controlled by the thermodynamics of a chemical reaction DH = Hproducts – Hreactants
- 10. Entropy, S, of a substance is a measure of its “disorder,” The greater the disorder, the greater the entropy. Gas has higher entropy than a liquid Ions in aqueous solution are normally more disordered than in their solid salt: ΔS is the change in entropy (entropy of products minus entropy of reactants) Free Energy A chemical reaction is driven toward the formation of products (the reaction is clearly favored) by a negative value of ΔH (heat given off) or a positive value of ΔS (more disorder) or both. When ΔH is positive and ΔS is negative, the reaction is clearly disfavored DS = Sproducts – Sreactants
- 11. Gibbs free energy, ΔG, A reaction is favored if ΔG is negative (At constant pressure) e.g. For the dissociation of HCl (favors) (disfavors)
- 12. ΔG is negative, so the reaction is favored when all species are in their standard state. The equilibrium constant depends on ΔG R is the gas constant 8.314 J/ (Kmol) T is temperature (Kelvin) For the last example
- 13. Because the equilibrium constant is large, HCl gas molecules are very soluble in water and are nearly completely ionized to cations and anions when dissolve in water. We say that a reaction is spontaneous under standard conditions if ΔG⁰ is negative Le Châtelier’s Principle If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position.
- 14. 14 Changes in Concentration N2 (g) + 3H2 (g) 2NH3 (g) Add NH3 Equilibrium shifts left to offset stress
- 15. Example
- 16. According to the principle of Le Châtelier, the reaction should go back to the left to partially offset the increase in dichromate, which appears on the right side of Reaction
- 17. When the temperature of a system is changed This term is independent of T
- 19. The equilibrium constant for the reaction in which a solid salt dissolves to give its constituent ions in solution (Solid is omitted from the equilibrium constant) e.g. Mercury (I) chloride (Hg2Cl2, also called mercurous chloride) The reaction in water is The solubility product constant, Ksp, is A saturated solution is one that contains excess, undissolved solid. The solution contains all the solid capable of being dissolved under the prevailing conditions.
- 21. 21 Molar solubility (mol/L) is the number of moles of solute dissolved in 1 L of a saturated solution. Solubility (g/L) is the number of grams of solute dissolved in 1 L of a saturated solution.
- 22. 22 What is the solubility of silver chloride in g/L? AgCl (s) Ag+ (aq) + Cl- (aq) Ksp = [Ag+][Cl-]Initial (M) Change (M) Equilibrium (M) 0.00 +s 0.00 +s s s Ksp = s2 s = Ksp s = 1.3 x 10-5 [Ag+] = 1.3 x 10-5 M [Cl-] = 1.3 x 10-5 M Solubility of AgCl = 1.3 x 10-5 mol AgCl 1 L soln 143.35 g AgCl 1 mol AgCl x = 1.9 x 10-3 g/L Ksp = 1.6 x 10-10 Example
- 24. 24 If 2.00 mL of 0.200 M NaOH are added to 1.00 L of 0.100 M CaCl2, will a precipitate form? The ions present in solution are Na+, OH-, Ca2+, Cl-. Only possible precipitate is Ca(OH)2 Is Q > Ksp for Ca(OH)2? [Ca2+]0 = 0.100 M [OH-]0 = 4.0 x 10-4 M Ksp = [Ca2+][OH-]2 = 8.0 x 10-6 Q = [Ca2+]0[OH-]0 2 = 0.100 x (4.0 x 10-4)2 = 1.6 x 10-8 Q < Ksp No precipitate will form Example Ca(OH)2 (s) Ca2+ (aq) + 2OH- (aq)
- 25. Exactly 200 mL of 0.0040 M BaCl2 are added to exactly 600 mL of 0.0080 M K2SO4. Will a precipitate form? Example
- 28. Example If the solubility of Ag2CrO4 (FM=332 g/mol) is 0.0279 g/L. Calculate Ksp? Solution [Ag2CrO4] = 0.0279 𝑔 𝐿 · 𝑚𝑜𝑙 332 𝑔 = 8.40 x 10-5 𝑚𝑜𝑙 𝐿 Ag2CrO4 2Ag+ (aq) + CrO4 2- (aq) Ksp 2S S Ksp = [Ag+]2 [CrO4 2-] = (2S)2 ∙ S = 4S3 = 4 (8.40 x 10-5 )3 = 2.37 x 10-12
- 29. Example Calculate the solubility of Ba(IO3)2 (FM=487 g/mol), Ksp=1.57 x 10-9 Ba(IO3)2 Ba2+ (aq) + 2IO3 - (aq) Ksp S 2S Solution Ksp = [Ba2+] [IO3 -]2 1.57 x 10-9 = (S) ∙ (2S)2 = 4S3 S3 = 1.57 x 10−9 4 = 3.93 x 10-10 S= (3.93 x 10-10)1/3 S = 7.32 x 10-4 mol/L (molar solubility) Solubility = 7.32 x 10-4 mol L · 487 g mol = 0.357 g L
- 30. This application of Le Chatelier’s principle is called the common ion effect. A salt will be less soluble if one of its constituent ions is already present in the solution. Common Ion Effect For the ionic solubility reaction
- 31. Example Calculate the solubility of Ba(IO3)2 in the presence of 0.10 M Ba(NO3)2. Ksp (Ba(IO3)2) = 1.57 x 10-9 Solution
- 33. Bronsted-Lowry definition does not require the formation of H3O+ (Extended to non-aqueous solutions or gas phase) Acid: proton donor Base: proton acceptor acid base salt Protic Acids and Bases The word protic refers to chemistry involving transfer of H+ from one molecule to another In aqueous chemistry: an acid is a substance that increases [H3O+] (hydronium ion) when added to water. (e.g. HCl in H2O). a base is a substance that decreases [H3O+] , therefore, a base increases [OH- ] in aqueous solution. (e.g. NaOH in H2O). HCl(g) + NH3(g) → NH4Cl(s) Most salts with a single positive and negative charge dissociate completely into ions in water
- 34. Conjugate Acids and Bases The products of a reaction between an acid and a base are also classified as acids and bases: Conjugate acids and bases are related by the gain or loss of one proton
- 35. The Nature of H+ and OH- The proton does not exist by itself in water. The simplest formula found in some crystalline salts is H3O+ For example, crystals of perchloric acid monohydrate contain pyramidal hydronium (also called hydroxonium) ions: A more accurate formula would be
- 36. In aqueous solution, H3O+ is tightly associated with three molecules of water through exceptionally strong hydrogen bonds (dotted lines), and one H2O (at the top) is held by weaker ion-dipole attraction (dashed line) To emphasize the chemistry of water, we will write H+ as H3O+ For example, water can be either an acid or a base. Water is an acid with respect to methoxide: But with respect to hydrogen bromide, water is a base:
- 37. Water undergoes self-ionization, called autoprotolysis (also called autoionization), in which it acts as both an acid and a base:
- 38. Protic solvents have a reactive H+ , and all protic solvents undergo autoprotolysis. Example: Acetic acid:
- 39. As the concentration of H+ increases, the concentration of OH - necessarily decreases, and vice versa. An approximate definition of pH is the negative logarithm of the H+ concentration
- 40. pH of various substances
- 41. Although pH generally falls in the range 0 to 14, these are not the limits of pH A pH of -1, for example, means -log[H+ ] = -1; or [H+ ] = 10 M This concentration is attained in a concentrated solution of a strong acid such as HCl.
- 42. Acids and bases are commonly classified as strong or weak, depending on whether they react nearly “completely” or only “partially” to produce H+ or OH- Even though the hydrogen halides HCl, HBr, and HI are strong acids, HF is not a strong acid. HF does completely give up its proton to H2O: Here the hydronium ion remains tightly associated with F- through a hydrogen bond, even in dilute solution Thus, HF does not behave as a strong acid
- 43. All weak acids, denoted HA, react with water by donating a proton to H2O: Dissociation of weak acid: The equilibrium constant is called Ka (the acid dissociation constant) A weak base, B, reacts with water by abstracting a proton from H2O: Base hydrolysis: The equilibrium constant Kb (the base hydrolysis constant)
- 44. Common Classes of Weak Acids and Bases Acetic acid is a typical weak acid; Most carboxylic acids are weak acids, and most carboxylate anions are weak bases.
- 45. Methylamine is a typical weak base; weak bases weak acids Amines are nitrogen-containing compounds:
- 46. Polyprotic acids and bases Compounds that can donate or accept more than one proton. e.g. Oxalic acid (diprotic)
- 48. Carbonic acid (H2CO3), a very important diprotic carboxylic acid is formed by the reaction of CO2 with H2O:
- 49. Relation Between Ka and Kb A relation between Ka and Kb of a conjugate acid-base pair in aqueous solution. e.g. The acid HA and its conjugate base A- ;
- 50. For a diprotic acid, we can derive relationships between each of two acids and their conjugate bases: The final results are
- 52. HW
- 53. HW Ksp of La(OH)3 is 2.0 x 10-21
- 54. → (Ksp of La(OH)3 is 2.0 x 10-21 ) Solution
- 57. Solution