Kinetics of enzyme action
•
38 gefällt mir•8,936 views
This presentation is about the kinetics of enzyme action , the Michaelis- Menten Model and kinetics of allosteric enzyme action in a simplified language.
Melden
Teilen
Melden
Teilen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Was ist angesagt?
Was ist angesagt? (20)
Regulatory and allosteric enzymes and allostrerism

Regulatory and allosteric enzymes and allostrerism
Kinetics of multi substrate enzyme catalyzed reaction

Kinetics of multi substrate enzyme catalyzed reaction
Mechanism of action of Chymotrypsin & Lysozyme.pptx

Mechanism of action of Chymotrypsin & Lysozyme.pptx
Enzyme kinetics- michaelis menten model, lineweaver burk plot

Enzyme kinetics- michaelis menten model, lineweaver burk plot
PYRUVATE DEHYDROGENASE COMPLEX (PDH-MULTI-ENZYME COMPLEX)

PYRUVATE DEHYDROGENASE COMPLEX (PDH-MULTI-ENZYME COMPLEX)
Ähnlich wie Kinetics of enzyme action
Dr.Anant Achary and Dr.S.Karthikumar
Department of Biotechnology
Kamaraj College of Engineering and Technology
S.P.G.C.Nagar, Near Virudhunagar, Tamilnadu
INDIA
Enzyme technology solved problems

Enzyme technology solved problemsDepartment of Biotechnology, Kamaraj college of engineering and technology
Ähnlich wie Kinetics of enzyme action (20)
7 29-10enzymeskinetics-coloso-110715062024-phpapp01[1]![7 29-10enzymeskinetics-coloso-110715062024-phpapp01[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![7 29-10enzymeskinetics-coloso-110715062024-phpapp01[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
7 29-10enzymeskinetics-coloso-110715062024-phpapp01[1]
Enzyme kinetics, factors and mechanism of enzyme activity

Enzyme kinetics, factors and mechanism of enzyme activity
Lineweaver - Burk Plot accurate determination of Vmax

Lineweaver - Burk Plot accurate determination of Vmax
Kürzlich hochgeladen
Kürzlich hochgeladen (20)
Recombination DNA Technology (Nucleic Acid Hybridization )

Recombination DNA Technology (Nucleic Acid Hybridization )
TEST BANK For Radiologic Science for Technologists, 12th Edition by Stewart C...

TEST BANK For Radiologic Science for Technologists, 12th Edition by Stewart C...
Raman spectroscopy.pptx M Pharm, M Sc, Advanced Spectral Analysis

Raman spectroscopy.pptx M Pharm, M Sc, Advanced Spectral Analysis
Lucknow 💋 Russian Call Girls Lucknow Finest Escorts Service 8923113531 Availa...

Lucknow 💋 Russian Call Girls Lucknow Finest Escorts Service 8923113531 Availa...
CALL ON ➥8923113531 🔝Call Girls Kesar Bagh Lucknow best Night Fun service 🪡

CALL ON ➥8923113531 🔝Call Girls Kesar Bagh Lucknow best Night Fun service 🪡
❤Jammu Kashmir Call Girls 8617697112 Personal Whatsapp Number 💦✅.

❤Jammu Kashmir Call Girls 8617697112 Personal Whatsapp Number 💦✅.
Stunning ➥8448380779▻ Call Girls In Panchshil Enclave Delhi NCR

Stunning ➥8448380779▻ Call Girls In Panchshil Enclave Delhi NCR
Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b

Nightside clouds and disequilibrium chemistry on the hot Jupiter WASP-43b
Discovery of an Accretion Streamer and a Slow Wide-angle Outflow around FUOri...

Discovery of an Accretion Streamer and a Slow Wide-angle Outflow around FUOri...
Chromatin Structure | EUCHROMATIN | HETEROCHROMATIN

Chromatin Structure | EUCHROMATIN | HETEROCHROMATIN
Physiochemical properties of nanomaterials and its nanotoxicity.pptx

Physiochemical properties of nanomaterials and its nanotoxicity.pptx
Forensic Biology & Its biological significance.pdf

Forensic Biology & Its biological significance.pdf
Hire 💕 9907093804 Hooghly Call Girls Service Call Girls Agency

Hire 💕 9907093804 Hooghly Call Girls Service Call Girls Agency
Pests of mustard_Identification_Management_Dr.UPR.pdf

Pests of mustard_Identification_Management_Dr.UPR.pdf
Kinetics of enzyme action
- 1. By Jyoti Verma M.Sc. Botany
- 2. Enzyme Kinetics- Enzyme kinetics is the study of enzymatic reaction rate and how it changes in response to different experimental parameters like substrate concentration and effect of inhibitors. It is an expression of chemical reactions in mathematical terms. It is an oldest approach to understand enzyme mechanism. History- • First began in 1902 when Andrian John Brown demonstrated the hydrolysis of sucrose by invertase. • The next important step of enzyme kinetics made by Leonor Michaelis and Maud Menten in 1913.They postulated the existence of intermediate complex.
- 3. Classification of Chemical Reactions- Order of Reaction- It is given by the sum of powers of concentrations. 1. Zero Order Reaction- The reaction in which the rate of reaction does not depends on the concentration of the substrate. R=K [S] 0 2. First Order Reaction- The reaction rate depends on substrate concentration and the sum of powers should be 1. R=K [A] 1 3. Second Order Reaction- A reaction is said to be second order when the sum of powers of concentration is 2. R=K[A] 1 [A] 1 R=K[A] 2
- 4. Michaelis - Menten Model of Enzyme Kinetics- In 1913 Leonor Michaelis and Maud Menten postulated the existence of enzyme substrate complex based on their observations on sucrose. They proposed- 1. The enzyme combines with substrate to form enzyme substrate complex (ES complex). 2. The substrate gets modified to form product and the product gets associated with enzyme (EP). 3. The product gets released from the enzyme. Leonor Michaelis Maud Menten
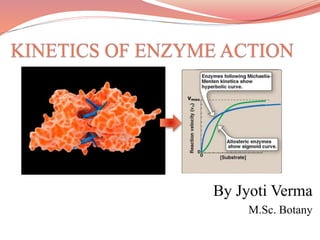
- 5. • They derived a relationship between the substrate concentration and the reaction rate. • As the substrate concentration increases the rate of reaction increases but upto a certain limit as all the enzymes gets saturated so further increase in substrate concentration does not have any effect. • When a graph is drawn between the substrate concentration [S] and Vo , a hyperbolic curve develops. • These observations can be expressed mathematically in the form of equation commonly known as Michaelis –Menten Equation. Michaelis –Menten Curve - A hyperbolic curve
- 6. The Michaelis - Menten Constant Km represents the concentration of substrate required to half saturate the enzyme. Km is a measure of the stability of the ES complex , being equal to the sum of the rates of breakdown of ES over its formation. Lower the Km more efficient the enzyme is ,as it requires very little amount of substrate to reach half Vmax while higher the Km less efficient the enzyme is, as it requires more substrate to reach half Vmax . Turnover Number and Catalytic Efficiency- • The Turnover number is the number of substrate molecule that are converted into product per unit time when the enzyme is saturated with the substrate. Kcat=Vmax/[E]T • Catalytic Efficiency = Kcat/Km • Increase in turnover number (Kcat) or decrease in Km results in enhanced catalytic efficiency.
- 7. Lineweaver – Burk Plot The Michaelis –Menten curve was not useful in determining the exact value of Vmax , So a more representating graph was suggested by Hans Lineweaver and Dean Burk. They employed a double reciprocal plot of 1/Vo versus 1/[S] from the Michaelis–Menten Equation. Advantage-More accurate determination of Vmax Lineweaver- Burk Plot
- 8. Kinetics of Enzyme Inhibition- Inhibitor- Any agent that decreases the velocity of an enzyme catalyzed reaction. Inhibition may be of reversible or non-reversible type. 1. Competitive Inhibitors- They compete directly with the substrate for the active site of the enzyme. This inhibition can be tackled by the addition of more substrate. Lineweaver- Burk Plot showing the effect of competitive inhibitors on enzyme action
- 9. Kinetics of Enzyme Inhibition- 2. Uncompetitive Inhibitors- These bind only to the Enzyme –Substrate complex , as binding of a substrate to enzyme creates a binding site for inhibitor. In this case Vmax and Km decreases .. Lineweaver- Burk Plot showing the effect of uncompetitive inhibitors on enzyme action
- 10. Kinetics of Enzyme Inhibition- 3. Non – Competitive Inhibitors- It can combine either with free substrate or the ES complex. They bind to a site other than active site of the enzyme. In this case Vmax decreases and Km remains unchanged. Lineweaver- Burk Plot showing the effect of non-competitive inhibitors on enzyme action
- 11. Kinetics of Allosteric enzymes- The allosteric enzymes have another site called allosteric site. Some molecule binding to allosteric site can act either as activator or inhibitor. They do not follow Michaelis-Menten kinetics instead of hyperbolic curve they show a sigmoidal curve because a small change in concentration will bring about a large change in reaction rate. Allosteric Enzyme
- 12. Significance of enzyme kinetics- With the help of enzyme kinetics we can determine the rate of the reaction with the changes in substrate concentration as well as we can determine the effect of inhibitors on reaction rate. It helps us to explain how enzyme works. Helps us to determine how drugs and poisons inhibit the enzyme activity. Helps us to understand enzyme’s role in metabolic pathway. It helps us to predict how enzymes behave in living organisms. THANKS