Trodelvy Any Chance Against Dato-DXd and Enhertu.pdf
•
0 likes•68 views
Here, we will discuss what are the advantages of Trodelvy, and which is superior in the field of breast cancer treatment, Trodelvy vs. Dato-DXd, Enhertu?
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
Vitamin D and calcium deficiency is associated with increased breast cancer risk and decreased breast cancer survival. The purpose of this study is to determine whether the addition of vit D and Zoledronic acid (ZA) to neoadjuvant chemotherapy (NACT) gives complete histological responses. We report a prospective evaluation comparing complete pathological response between different biomolecular sub-groups.Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...

Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...CrimsonpublishersCancer
More Related Content
Similar to Trodelvy Any Chance Against Dato-DXd and Enhertu.pdf
Vitamin D and calcium deficiency is associated with increased breast cancer risk and decreased breast cancer survival. The purpose of this study is to determine whether the addition of vit D and Zoledronic acid (ZA) to neoadjuvant chemotherapy (NACT) gives complete histological responses. We report a prospective evaluation comparing complete pathological response between different biomolecular sub-groups.Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...

Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...CrimsonpublishersCancer
Similar to Trodelvy Any Chance Against Dato-DXd and Enhertu.pdf (20)
Advances in triple negative breast cancer (tnbc) targeted therapy drugs

Advances in triple negative breast cancer (tnbc) targeted therapy drugs
A Look into Antibody–drug conjugates (ADCs) Targets.pdf

A Look into Antibody–drug conjugates (ADCs) Targets.pdf
Peptide drug conjugates (pd cs) new generation of targeted cancer treatment

Peptide drug conjugates (pd cs) new generation of targeted cancer treatment
Positive Phase II results for trastuzumab emtansine (T-DM1)

Positive Phase II results for trastuzumab emtansine (T-DM1)
Clinical Development of ADC Drugs Targeting TROP-2.pdf

Clinical Development of ADC Drugs Targeting TROP-2.pdf
New Oncology Trends ADCs, Bispecific Antibodies & CAR-T Cell.pdf

New Oncology Trends ADCs, Bispecific Antibodies & CAR-T Cell.pdf
Key Slides on Individualizing ART Management Based on Treatment Safety and To...

Key Slides on Individualizing ART Management Based on Treatment Safety and To...
DS-8201 (Enhertu) A Potential ADC Drug Targeting HER2.pdf

DS-8201 (Enhertu) A Potential ADC Drug Targeting HER2.pdf
Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...

Vitamin D and Biphosphonate in Neoadjuvant Advanced Breast Cancer_Crimson Pub...
CCO Rational Options in Breast Cancer : How Molecular Understanding Informs T...

CCO Rational Options in Breast Cancer : How Molecular Understanding Informs T...
More from DoriaFang
More from DoriaFang (20)
Cyclic Peptides Current Status & Future Prospects.pdf

Cyclic Peptides Current Status & Future Prospects.pdf
Antibody–Oligonucleotide Conjugates (AOCs) in Clinical Trials.pdf

Antibody–Oligonucleotide Conjugates (AOCs) in Clinical Trials.pdf
Alzheimer's Disease Drug Development Aducanumab, Lecanemab & Donanemab.pdf

Alzheimer's Disease Drug Development Aducanumab, Lecanemab & Donanemab.pdf
Claudin6 (CLDN6) A Emerging Target For Solid Tumor.pdf

Claudin6 (CLDN6) A Emerging Target For Solid Tumor.pdf
ROR1 ADCs in Clinical Trials MK-2140, NBE-002 & CS5001.pdf

ROR1 ADCs in Clinical Trials MK-2140, NBE-002 & CS5001.pdf
Summary of Targeted Protein Degradation in Clinical Trials.pdf

Summary of Targeted Protein Degradation in Clinical Trials.pdf
Trophoblast Glycoprotein (TPGB5T4) A New Target For ADC Drugs.pdf

Trophoblast Glycoprotein (TPGB5T4) A New Target For ADC Drugs.pdf
Overview of Oral Delivery Strategies for Peptides.pdf

Overview of Oral Delivery Strategies for Peptides.pdf
Summary of ADC Targets For Solid Tumors & Hematological Tumors.pdf

Summary of ADC Targets For Solid Tumors & Hematological Tumors.pdf
Bispecific Antibody-drug Conjugate Drugs In Clinical or Preclinical.pdf

Bispecific Antibody-drug Conjugate Drugs In Clinical or Preclinical.pdf
Nectin-4 New Antibody-Drug Conjugate (ADC) Target.pdf

Nectin-4 New Antibody-Drug Conjugate (ADC) Target.pdf
PROTAC Delivery System Recent Research Advances.pdf

PROTAC Delivery System Recent Research Advances.pdf
Aptamer-Drug Conjugate (ApDC) Current Research Progress.pdf

Aptamer-Drug Conjugate (ApDC) Current Research Progress.pdf
Summary of Molecular Glues Approved or in Clinical Trial.pdf

Summary of Molecular Glues Approved or in Clinical Trial.pdf
Recently uploaded
Saudi Arabia [ Abortion pills) Jeddah/riaydh/dammam/++918133066128☎️] cytotec tablets uses abortion pills 💊💊 How effective is the abortion pill? 💊💊 +918133066128) "Abortion pills in Jeddah" how to get cytotec tablets in Riyadh " Abortion pills in dammam*💊💊 The abortion pill is very effective. If you’re taking mifepristone and misoprostol, it depends on how far along the pregnancy is, and how many doses of medicine you take:💊💊 +918133066128) how to buy cytotec pills
At 8 weeks pregnant or less, it works about 94-98% of the time. +918133066128[ 💊💊💊 At 8-9 weeks pregnant, it works about 94-96% of the time. +918133066128) At 9-10 weeks pregnant, it works about 91-93% of the time. +918133066128)💊💊 If you take an extra dose of misoprostol, it works about 99% of the time. At 10-11 weeks pregnant, it works about 87% of the time. +918133066128) If you take an extra dose of misoprostol, it works about 98% of the time. In general, taking both mifepristone and+918133066128 misoprostol works a bit better than taking misoprostol only. +918133066128 Taking misoprostol alone works to end the+918133066128 pregnancy about 85-95% of the time — depending on how far along the+918133066128 pregnancy is and how you take the medicine. +918133066128 The abortion pill usually works, but if it doesn’t, you can take more medicine or have an in-clinic abortion. +918133066128 When can I take the abortion pill?+918133066128 In general, you can have a medication abortion up to 77 days (11 weeks)+918133066128 after the first day of your last period. If it’s been 78 days or more since the first day of your last+918133066128 period, you can have an in-clinic abortion to end your pregnancy.+918133066128
Why do people choose the abortion pill? Which kind of abortion you choose all depends on your personal+918133066128 preference and situation. With+918133066128 medication+918133066128 abortion, some people like that you don’t need to have a procedure in a doctor’s office. You can have your medication abortion on your own+918133066128 schedule, at home or in another comfortable place that you choose.+918133066128 You get to decide who you want to be with during your abortion, or you can go it alone. Because+918133066128 medication abortion is similar to a miscarriage, many people feel like it’s more “natural” and less invasive. And some+918133066128 people may not have an in-clinic abortion provider close by, so abortion pills are more available to+918133066128 them. +918133066128 Your doctor, nurse, or health center staff can help you decide which kind of abortion is best for you. +918133066128 More questions from patients: Saudi Arabia+918133066128 CYTOTEC Misoprostol Tablets. Misoprostol is a medication that can prevent stomach ulcers if you also take NSAID medications. It reduces the amount of acid in your stomach, which protects your stomach lining. The brand name of this medication is Cytotec®.+918133066128) Unwanted Kit is a combination of two medicines, ounwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi![unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE AbudhabiAbortion pills in Kuwait Cytotec pills in Kuwait
VIP Call Girls Napur Anamika Call Now: 8617697112 Napur Escorts Booking Contact Details WhatsApp Chat: +91-8617697112 Napur Escort Service includes providing maximum physical satisfaction to their clients as well as engaging conversation that keeps your time enjoyable and entertaining. Plus they look fabulously elegant; making an impressionable. Independent Escorts Napur understands the value of confidentiality and discretion - they will go the extra mile to meet your needs. Simply contact them via text messaging or through their online profiles; they'd be more than delighted to accommodate any request or arrange a romantic date or fun-filled night together. We provide –(Anamika) VIP Call Girls Napur Call Now 8617697112 Napur Escorts 24x7

(Anamika) VIP Call Girls Napur Call Now 8617697112 Napur Escorts 24x7Call Girls in Nagpur High Profile Call Girls
Recently uploaded (20)
RSA Conference Exhibitor List 2024 - Exhibitors Data

RSA Conference Exhibitor List 2024 - Exhibitors Data
Quick Doctor In Kuwait +2773`7758`557 Kuwait Doha Qatar Dubai Abu Dhabi Sharj...

Quick Doctor In Kuwait +2773`7758`557 Kuwait Doha Qatar Dubai Abu Dhabi Sharj...
Call Girls Zirakpur👧 Book Now📱7837612180 📞👉Call Girl Service In Zirakpur No A...

Call Girls Zirakpur👧 Book Now📱7837612180 📞👉Call Girl Service In Zirakpur No A...
unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi![unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
unwanted pregnancy Kit [+918133066128] Abortion Pills IN Dubai UAE Abudhabi
FULL ENJOY Call Girls In Majnu Ka Tilla, Delhi Contact Us 8377877756

FULL ENJOY Call Girls In Majnu Ka Tilla, Delhi Contact Us 8377877756
👉Chandigarh Call Girls 👉9878799926👉Just Call👉Chandigarh Call Girl In Chandiga...

👉Chandigarh Call Girls 👉9878799926👉Just Call👉Chandigarh Call Girl In Chandiga...
Eluru Call Girls Service ☎ ️93326-06886 ❤️🔥 Enjoy 24/7 Escort Service

Eluru Call Girls Service ☎ ️93326-06886 ❤️🔥 Enjoy 24/7 Escort Service
Call Girls Kengeri Satellite Town Just Call 👗 7737669865 👗 Top Class Call Gir...

Call Girls Kengeri Satellite Town Just Call 👗 7737669865 👗 Top Class Call Gir...
Call Girls Jp Nagar Just Call 👗 7737669865 👗 Top Class Call Girl Service Bang...

Call Girls Jp Nagar Just Call 👗 7737669865 👗 Top Class Call Girl Service Bang...
Falcon's Invoice Discounting: Your Path to Prosperity

Falcon's Invoice Discounting: Your Path to Prosperity
Russian Call Girls In Gurgaon ❤️8448577510 ⊹Best Escorts Service In 24/7 Delh...

Russian Call Girls In Gurgaon ❤️8448577510 ⊹Best Escorts Service In 24/7 Delh...
Call Girls From Pari Chowk Greater Noida ❤️8448577510 ⊹Best Escorts Service I...

Call Girls From Pari Chowk Greater Noida ❤️8448577510 ⊹Best Escorts Service I...
BAGALUR CALL GIRL IN 98274*61493 ❤CALL GIRLS IN ESCORT SERVICE❤CALL GIRL

BAGALUR CALL GIRL IN 98274*61493 ❤CALL GIRLS IN ESCORT SERVICE❤CALL GIRL
(Anamika) VIP Call Girls Napur Call Now 8617697112 Napur Escorts 24x7

(Anamika) VIP Call Girls Napur Call Now 8617697112 Napur Escorts 24x7
MONA 98765-12871 CALL GIRLS IN LUDHIANA LUDHIANA CALL GIRL

MONA 98765-12871 CALL GIRLS IN LUDHIANA LUDHIANA CALL GIRL
Trodelvy Any Chance Against Dato-DXd and Enhertu.pdf
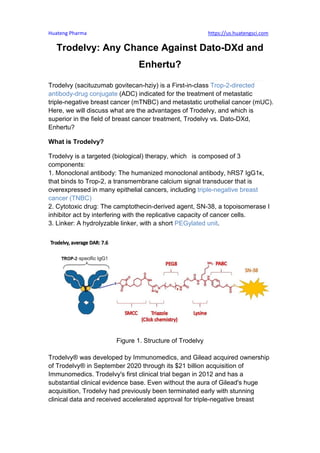
- 1. Huateng Pharma https://us.huatengsci.com Trodelvy: Any Chance Against Dato-DXd and Enhertu? Trodelvy (sacituzumab govitecan-hziy) is a First-in-class Trop-2-directed antibody-drug conjugate (ADC) indicated for the treatment of metastatic triple-negative breast cancer (mTNBC) and metastatic urothelial cancer (mUC). Here, we will discuss what are the advantages of Trodelvy, and which is superior in the field of breast cancer treatment, Trodelvy vs. Dato-DXd, Enhertu? What is Trodelvy? Trodelvy is a targeted (biological) therapy, which is composed of 3 components: 1. Monoclonal antibody: The humanized monoclonal antibody, hRS7 IgG1κ, that binds to Trop-2, a transmembrane calcium signal transducer that is overexpressed in many epithelial cancers, including triple-negative breast cancer (TNBC) 2. Cytotoxic drug: The camptothecin-derived agent, SN-38, a topoisomerase I inhibitor act by interfering with the replicative capacity of cancer cells. 3. Linker: A hydrolyzable linker, with a short PEGylated unit. Figure 1. Structure of Trodelvy Trodelvy® was developed by Immunomedics, and Gilead acquired ownership of Trodelvy® in September 2020 through its $21 billion acquisition of Immunomedics. Trodelvy's first clinical trial began in 2012 and has a substantial clinical evidence base. Even without the aura of Gilead's huge acquisition, Trodelvy had previously been terminated early with stunning clinical data and received accelerated approval for triple-negative breast
- 2. Huateng Pharma https://us.huatengsci.com cancer (TNBC) nearly 2 months ahead of the scheduled approval date, making it a potential star drug. Trodelvy has been approved in more than 35 countries and is undergoing multiple additional regulatory reviews worldwide for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received two or more prior systemic therapies. Gilead Sciences has submitted a supplemental Biologics License Application to the U.S. FDA. In addition to breast cancer, Trodelvy® has shown positive performance in several cancer types including uroepithelial cancer, lung cancer, and prostate cancer. Phase II clinical studies for single-agent second-line treatment of uroepithelial carcinoma showed an ORR of 28%, and this indication has been approved for marketing by the FDA.
- 3. Huateng Pharma https://us.huatengsci.com Figure 2. Pipelines of Trodelvy, source: Gilead official website Trodelvy vs. Dato-DXd, Enhertu Trodelvy's sales were $380 million in 2021. According to analysts, Trodelvy's sales need to peak at $4 billion in order for Gilead to recover the cost of the acquisition. But the potential competition is also fierce and challenging for Trodelvy's subsequent growth. Currently, the two products with the most competition are Dato-DXd and Enhertu. Dato-DXd In terms of same-target ADC products, there are more than 20 TROP-2 ADCs in development worldwide. Its main competitor, Datopotamab deruxtecan (DS-1062/Dato-DXd) from Daiichi Sankyo/AstraZeneca, is already in Phase III clinical development. Figure 3. Stucture of Dato-DXd, and comparison between Dato-DXd & Trodelvy
- 4. Huateng Pharma https://us.huatengsci.com Dato-DXd is designed and developed based on Daiichi Sankyo's proprietary DXd-ADC technology platform, which uses DNA topoisomerase I to inhibit DXd with high activity and is able to kill nearby cancer cells through the "bystander effect" with short half-life and low toxic side effects. The linker is highly stable and can be specifically cleaved by enzymes in the tumor tissue, avoiding the killing effect on normal tissue. On December 7, 2021, Daiichi Sankyo disclosed at SABCS the latest data from the TROPION-PanTumor01 TNBC cohort, a clinical phase I trial of Dato-DXd, showing that Dato-DXd demonstrated sustained remission and disease control in patients with metastatic triple-negative breast cancer who experienced disease progression after standard therapy. Specific results showed that of the 44 patients treated with Dato-DXd (6 mg/kg [n=42] and 8 mg/kg [n=2]) 15 patients achieved objective remission with an ORR of 34%. After a median follow-up of 7.6 months (4-13 months), 14 patients were confirmed to have achieved complete/partial remission, with a median duration of remission not yet reached as most patients remained in remission, for a disease control rate of 77%. In the subgroup of 27 patients with measurable disease and not previously treated with topoisomerase I inhibitor-ADC drugs, the ORR in the Dato-DXd group was 52%, and after a median follow-up of 8.8 months (4-13 months), 13 patients were confirmed to have achieved CR/PRs, 1 patient with CR/PRs yet to be confirmed, and another 9 patients had stable disease. The DCR for this subgroup of patients was 81%. With regard to safety, treatment-related adverse events occurred in ≥15% of patients including: nausea, stomatitis, vomiting, fatigue, hair loss, mucosal inflammation, constipation, headache, lymphopenia, neutropenia, fever, anemia, pruritus, hypokalemia, diarrhea, and cough. 23% of patients experienced ≥ grade 3 treatment-related adverse events. There were no cases of interstitial lung disease (ILD). Enhertu One of Trodelvy's indications, HR+/HER2- breast cancer, has been challenged by a strong rival, Enhertu, although the patient populations are not the same, but there is some degree of overlap. Trodelvy's Phase III TROPiCS-02 study included patients with HR+/HER2- metastatic breast cancer who had received endocrine therapy, CDK4/6 inhibitors and 2-4 lines of chemotherapy, including patients with HER2-low and IHC 0 status. At the 2022 ASCO meeting, data from Enhertu's DESTINY-Breast04 and Trodelvy's TROPiCS-02 were both released. DESTINY-Breast04, which defines a new breast cancer staging that distinguishes low HER2 expression (IHC 1+ or IHC2+/ISH negative) from the HER2-negative population, was approved by the FDA for priority review in approximately two weeks. TROPiCS-02, despite meeting clinical endpoints and being included in the
- 5. Huateng Pharma https://us.huatengsci.com NCCN guidelines as a Class 2A recommendation based on positive data, did not yield the expected results. Detailed data can be found in the chart below. Figure 4. Tropics-02: KM-curve for progression free survival (primary endpoint) Figure 5. DESTINY-Breast04 results
- 6. Huateng Pharma https://us.huatengsci.com Huateng Pharma is dedicated to being your most reliable partner to provide chemical synthesis and high-quality PEG linkers for ADC drugs. We are committed to promoting the progress of your ADC discovery and development projects. References: [1] Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021;384(16):1529-1541. doi:10.1056/NEJMoa2028485 [2]https://www.cancernetwork.com/view/positive-response-activity-seen-with-d atopotamab-deruxtecan-in-advanced-tnbc [3] https://www.medscape.com/viewarticle/974274 Related Articles: A Look into Antibody–drug conjugates (ADCs) Targets Overview of Triple Negative Breast Cancer And FDA Approved Therapies ADC Linker - Development and Challenges What Are PEG Linkers and Their Applications?