Key CRC data from ASCO 2013: FIRE-3, CAIRO3 and more
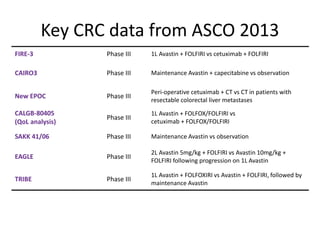
- 1. Key CRC data from ASCO 2013 FIRE-3 Phase III 1L Avastin + FOLFIRI vs cetuximab + FOLFIRI CAIRO3 Phase III Maintenance Avastin + capecitabine vs observation New EPOC Phase III Peri-operative cetuximab + CT vs CT in patients with resectable colorectal liver metastases CALGB-80405 (QoL analysis) Phase III 1L Avastin + FOLFOX/FOLFIRI vs cetuximab + FOLFOX/FOLFIRI SAKK 41/06 Phase III Maintenance Avastin vs observation EAGLE Phase III 2L Avastin 5mg/kg + FOLFIRI vs Avastin 10mg/kg + FOLFIRI following progression on 1L Avastin TRIBE Phase III 1L Avastin + FOLFOXIRI vs Avastin + FOLFIRI, followed by maintenance Avastin
- 3. PEAK (KRAS/NRAS analysis) Phase II 1L Avastin + mFOLFOX6 vs panitumumab + mFOLFOX6 PRIME (KRAS/NRAS analysis) Phase III 1L panitumumab + FOLFOX4 vs FOLFOX4 PRIME (by KRAS exon 2 status ) Phase III 1L panitumumab + FOLFOX4 vs FOLFOX4 DREAM Phase III Maintenance Avastin ± erlotinib AVEX Phase III 1L Avastin + capecitabine vs capecitabine in elderly mCRC patients TML Phase III Avastin + chemotherapy beyond first progression vs chemotherapy OLIVIA Phase II Avastin + mFOLFOX6 vs Avastin + FOLFOXIRI in initially unresectable liver-limited mCRC SOFT Phase III S-1/oxaliplatin (SOX) + Avastin vs mFOLFOX6 + Avastin Key CRC data from ASCO 2013 (cont’d)
- 4. FIRE-3
- 5. FIRE-3: phase III H2H trial comparing 1L Avastin + FOLFIRI with cetuximab + FOLFIRI Heinemann, et al. ASCO 2013 Previously untreated KRAS WT mCRC (n=592) Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) R • Phase III • Primary endpoint: ORR • Secondary endpoints: PFS, OS, time to failure of first-line therapy, ‘deepness of response’ (% tumour shrinkage compared to baseline), secondary R0 resection rate, safety
- 6. Cetuximab + FOLFIRI Avastin + FOLFIRI ITT population (n=592) (n=297) (n=295) ORR (95% CI), % 62.0 (56.2–67.5) 58.0 (52.1–63.7) Odds ratio 1.18 (0.85-1.64) p valueǂ 0.183 CR 4.4 1.4 PR 57.6 56.6 SD 17.5 28.8 PD 7.1 5.4 Not evaluable 13.1 7.8 FIRE-3: no significant increase in the primary endpoint of ORR* with cetuximab-based therapy *ITT population; investigator-reported; ǂFisher’s exact test (one-sided) Heinemann, et al. ASCO 2013 • With a p value of 0.183, the futility of the primary analysis was substantial • The main reason for missing the primary endpoint was the higher than expected ORR in the Avastin arm
- 7. ORR subgroup data in patients assessable for response1 Assessable for response (n=526) Cetuximab + FOLFIRI (n=255) Avastin + FOLFIRI (n=271) ORR (95% CI), % 72.2 (66.2–77.6) 63.1 (57.1–68.9) Odds ratio 1.52 (1.05-2.19) p-value* 0.017 *Fisher’s exact test (one-sided) 1. Heinemann, et al. ASCO 2013; 2. Bergsland, et al. ASCO 2013 • The ‘assessable for response’ subgroup includes patients who had at least one additional CT scan to compare with their baseline scan in order to measure response and at least three completed cycles of chemotherapy • The significant increase in ORR in patients assessable for response should be interpreted with caution as the number of patients excluded was significantly different in each treatment arm (n=42 in cetuximab arm vs n=24 in Avastin arm, p=0.026 [2-sided Fisher’s exact test]); of the 42 patients excluded from the cetuximab arm, 13 patients were excluded due to allergic reaction, 28 for ‘other reasons’ and one for early death2 ORR in the assessable for response subgroup was not defined as the primary endpoint; the study was designed and powered to show superiority of cetuximab over Avastin in the ITT population
- 8. Patients at risk 297 100 19 10 5 3 295 99 15 6 4 FIRE-3: PFS was similar with Avastin- and cetuximab-based therapy PFSestimate 1.0 0.75 0.50 0.25 0 Heinemann, et al. ASCO 2013 Cetuximab + FOLFIRI Avastin + FOLFIRI Events, n/N (%) 250/297 (84.2) 242/295 (82.0) Median, months 10.0 10.3 HR (95% CI) p value 1.06 (0.88–1.26) p=0.547 10.0 10.3 Time (months) 0 12 24 36 48 60 72
- 9. Patients at risk 297 218 111 60 29 9 295 214 111 47 18 2 FIRE-3: OS (secondary endpoint) higher in FOLFIRI/cetuximab arm OSestimate 1.0 0.75 0.50 Time (months) 0 12 24 36 48 60 72 Median duration of treatment = 5 months (all 3 agents) Median PFS = 10.0 months Cetuximab + FOLFIRI Avastin + FOLFIRI Events, n/N (%) 158/297 (53.2) 185/295 (62.7) Median, months 28.7 25.0 HR (95% CI) p value 0.77 (0.62–0.96) p=0.017 Median follow-up >30 months in both treatment arms; Heinemann, et al. ASCO 2013 The separation of OS curves observed at ~24 months is highly unlikely to be attributable to the first-line treatment effect of ~5 months of biological treatment 0.25 0
- 10. Subsequent anti-cancer therapy Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) p value* Any second-line therapy, % 65.7 61.7 0.347 Second-line Avastin, % 48.2 17.6 Second-line anti-EGFR therapy, % 14.4 42.9 *Two-sided Fisher exact test Heinemann, et al. ASCO 2013
- 11. FIRE-3: no difference in haematological toxicity between treatment arms Heinemann, et al. ASCO 2013 Adverse event, % Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) p value (grade ≥3)Any grade Grade ≥3 Any grade Grade ≥3 Leucopenia 66.7 12.8 66.8 11.2 0.613 Anaemia 87.9 2.4 90.9 1.4 0.545 Thrombocytopenia 25.6 0.3 23.4 0.3 >0.999 Neutropenia 61.3 24.2 60.3 22.8 0.699 Febrile neutropenia 1.7 1.7 3.0 1.0 0.725
- 12. FIRE-3: no major difference in non-haematological toxicity between treatment arms Adverse event, % Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) p value (grade ≥3)Any grade Grade ≥3 Any grade Grade ≥3 Any adverse event 100.0 71.0 100.0 63.7 0.066 Nausea 48.2* 3.4 62.4* 4.8 0.414 Vomiting 24.6ǂ 2.4 32.9ǂ 3.4 0.473 Diarrhoea 57.2 11.5 62.7 13.6 0.458 Mucositis/stomatitis 42.1 3.7 44.8 4.1 0.835 Fatigue 50.2 0.7 54.9 1.4 0.449 Pain 50.2 5.4 58.0 7.1 0.401 Hand-foot syndrome 26.6§ 3.4 14.2§ 0.7 0.037 Fatal adverse events N/A 0.0 N/A 1.7 0.030 Heinemann, et al. ASCO 2013 Significant differences in any-grade toxicity: *p=0.0005; ǂp=0.03; §p=0.0002
- 13. FIRE-3: significant increase in grade ≥3 adverse events of special interest to cetuximab Heinemann, et al. ASCO 2013 Adverse event, % Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) p value (grade ≥3)Any grade Grade ≥3 Any grade Grade ≥3 Acneiform exanthema 77.4* 16.8 7.8* 0.0 <0.0001 Desquamation 35.4* 6.7 11.5* 0.7 0.0001 Paronychia 37.4* 5.7 9.2* 0.0 <0.0001 Infusion-related allergic reaction 7.7* 4.0 0.0* 0.0 0.0004 Hypocalcaemia 27.6ǂ 4.0 15.3ǂ 2.4 0.351 Hypomagnesaemia 63.3* 4.4 39.7* 0.7 0.007 Significant differences in any-grade toxicity: *p<0.0001; ǂp=0.0003 Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5% • Cetuximab resulted in significantly increased grade ≥3 adverse events of special interest to anti-EGFR therapy
- 14. FIRE-3: no significant difference in grade ≥3 adverse events of special interest to Avastin Heinemann, et al. ASCO 2013 Significant differences in any-grade toxicity: *p<0.001; ǂp=0.046; §p=0.006 Adverse event, % Cetuximab + FOLFIRI (n=297) Avastin + FOLFIRI (n=295) p value (grade ≥3)Any grade Grade ≥3 Any grade Grade ≥3 Hypertension 21.2* 6.4 38.3* 6.8 0.870 Proteinuria 2.7 0 2.0 0.3 0.498 Bleeding 21.2ǂ 0.7 28.5ǂ 0.3 >0.999 Abscess/fistula 1.4§ 0.3 5.4§ 1.0 0.372 GI perforation 0.3 0.3 0.7 0.7 0.623 Thrombosis (any) 9.4 6.1 11.5 6.1 >0.999 Thromboembolic event 7.4 5.1 7.1 5.8 0.720 Wound healing complications 2.0 0.3 2.7 1.4 0.216 • Avastin did not lead to any significant difference in grade ≥3 adverse events of special interest to anti-VEGF treatment
- 15. CAIRO3
- 16. CAIRO3: maintenance Avastin + capecitabine versus observation Koopman, et al. ASCO 2013 • Phase III trial • Primary endpoint: PFS after re-introduction = PFS2 • Secondary endpoints: PFS1, OS, TTP2, ORR, safety • PFS2 was considered to be equal to PFS1 for patients in whom Avastin + XELOX was not reintroduced after PFS1 for any reason • Upon PD1, 75% of patients received Avastin + XELOX in arm A and 47% in arm B Previously untreated mCRC (n=558) R Avastin + XELOX (x6) CR PR SD Avastin + capecitabine Observation Avastin + XELOX PD2PD1 PFS2 PFS1 TTP2 Arm A Arm B Avastin + XELOX PD2PD1
- 17. CAIRO3: study profile Data cut-off 190413; median duration of follow-up 40 months Koopman, et al. ASCO 2013 558 patients enrolled 279 patients observation 279 patients maintenance 212 patients (76%) Avastin + XELOX 67 patients (24%) • Ongoing observation • No treatment • Other treatment 131 patients (47%) Avastin + XELOX 148 patients (53%) • Ongoing maintenance • No treatment • Other treatment
- 18. CAIRO3: PFS1 significantly improved with maintenance AvastinPFS1estimate 279 85 18 9 6 6 3Observation 279 172 89 44 29 15 9Maintenance *Adjusted for covariates with imbalances at baseline; Koopman, et al. ASCO 2013 Maintenance Observation Median PFS1, months 8.5 4.1 Stratified HR (95% CI) 0.44 (0.36–0.53) p<0.00001 Adjusted* HR 0.41 p <0.001 4.1 8.5 0 6 12 18 24 30 36 0.0 0.2 0.4 0.6 0.8 1.0 Time (months)
- 19. CAIRO3: PFS2 significantly improved with maintenance Avastin Time (months) PFS2estimate 0 6 12 18 24 30 36 0.0 0.2 0.4 0.6 0.8 1.0 279 207 111 42 16 11 4Observation 279 207 130 66 38 23 12Maintenance 10.5 11.8 Maintenance Observation Median PFS2, months 11.8 10.5 Stratified HR (95% CI) 0.81 (0.67–0.98) p=0.028 Adjusted* HR 0.77 p=0.007 *Adjusted for covariates with imbalances at baseline; Koopman, et al. ASCO 2013
- 20. Time (months) OSestimate 0 6 12 18 24 30 36 0.0 0.2 0.4 0.6 0.8 1.0 279 248 184 122 78 53 28Observation 279 252 192 143 95 58 33Maintenance CAIRO3: OS significantly improved with maintenance Avastin (preliminary analysis) Maintenance Observation Median OS, months 21.7 18.2 Stratified HR (95% CI) 0.87 (0.71–1.06) p=0.156 Adjusted* HR 0.80 p=0.035 18.2 21.7 *Adjusted for covariates with imbalances at baseline; Koopman, et al. ASCO 2013
- 21. CAIRO3: safety profile during observation/ maintenance Koopman, et al. ASCO 2013 Grade 3/4 adverse event, % Observation (n=279) Maintenance (n=279) Hypertension 18 24 Neutropenia 0 2 Thrombocytopenia 0 1 Diarrhoea 1 3 Vomiting 1 0.4 Nausea 0 2 Hand-foot syndrome 0 22 Neurotoxicity 5 10 GI perforation 0 1 Venous thromboembolic events 2 3 Fatigue 2 4 Red box indicates a difference in incidence between treatment arms of ≥5%
- 22. New EPOC
- 23. • Phase III • Primary endpoint: PFS • Secondary endpoints: OS, pre-operative response, pathological resection status, peri-operative safety findings, QoL, measures for cost-effectiveness • New EPOC is an extension to the EPOC study, which randomised patients to surgery alone versus surgery + chemotherapy Following IDMC recommendation, new EPOC was terminated early when the study met a protocol predefined futility analysis (after 123 of the required 212 expected events had occurred, with 272 patients) New EPOC: peri-operative cetuximab + CT vs CT alone CT = FOLFOX4, XELOX or FOLFIRI Primrose, et al. ASCO 2013 Resectable or borderline resectable colorectal liver mets and KRAS WT mCRC (n=272) CT alone 12 weeks (n=134) Cetuximab + CT 12 weeks (n=137) R Surgery Surgery CT alone 12 weeks (n=134) Cetuximab + CT 12 weeks (n=137)
- 24. New EPOC: significantly poorer PFS with cetuximab + CT compared to CT alone Cetuximab + CT (n=116) CT alone (n=117) Median, months 14.1 20.5 HR (95% CI) p value 1.49 (1.04–2.12) 0.030 Patients at risk CT aIone 116 89 65 38 23 12 5 2 1 1 0 Cetuximab + CT 117 87 54 24 15 5 3 2 1 0 0 PFSestimate 1.0 0.75 0.5 0.25 0 Time (months) 0 6 12 18 24 30 36 42 48 54 60 14.1 20.5 The primary endpoint of PFS was not met; cetuximab-based therapy resulted in significant detrimental effect on PFS Primrose, et al. ASCO 2013; CT = FOLFOX4, XELOX or FOLFIRI
- 25. 0 6 12 18 24 30 36 42 48 54 60 CT alone (arm A) Cetuximab + CT (arm B) Median OS, months NR 39.1 HR (95% CI) p value 1.48 (0.85‒2.58) 0.163 CT = FOLFOX4, XELOX or FOLFIRI; NR = not reached Primrose, et al. ASCO 2013 39.1 NR Patients at risk CT aIone 127 113 90 61 40 29 12 4 2 1 0 Cetuximab + CT 127 99 81 55 38 22 7 2 1 0 0 New EPOC: numerically shorter OS with cetuximab + CT compared to CT alone OSestimate 1.0 0.75 0.5 0.25 0 Time (months)
- 26. New EPOC: safety Grade ≥3 adverse event, % CT alone (n=134) Cetuximab + CT (n=137) Pre-operative chemotherapy Overall 40.3 46.7 Nausea/vomiting 3.0 4.4 Skin rash 1.5 15.3 Peripheral neuropathy 4.5 0.7 Hypomagnesaemia 0 1.5 Embolic event 4.5 5.8 Post-operative chemotherapy Overall 21.2 27.9 Nausea/vomiting 3.8 1.9 Skin rash 0 7.7 Peripheral neuropathy 1.9 3.8 Hypomagnesaemia 0 0 Embolic event 1.9 2.9 CT = FOLFOX4, XELOX or FOLFIRI Primrose, et al. ASCO 2013 Red box indicates a difference in incidence between treatment arms of ≥5%
- 28. CALGB 80405 QoL analysis comparing 1L cetuximab vs Avastin, in combination with FOLFOX/FOLFIRI Previously untreated KRAS WT mCRC (n=2,900) (n=518 in QoL analysis) *Use of FOLFOX or FOLFIRI was at the physician’s discretion Naughton, et al. ASCO 2013 • Phase III • Primary endpoint: QoL at 3 months • QoL was assessed at baseline, 6 weeks, and 3, 6 and 9 months post-randomisation, using the EORTC QLQ-30 and the Dermatology- Specific Quality of Life (DSQL) scales • As the QoL analysis enrolled the first 518 patients randomised to CALGB 80405, the majority were enrolled prior to a protocol amendment eliminating the dual biologic arm and restricting participation to patients with KRAS WT tumours Cetuximab + FOLFOX/FOLFIRI* Avastin + FOLFOX/FOLFIRI* R Cetuximab + Avastin + FOLFOX/FOLFIRI* A protocol amendment meant that this dual biologic arm was eliminated during trial
- 29. CALGB 80405: cetuximab-associated skin toxicity impacts on QoL Avastin + FOLFOX/FOLFIRI Cetuximab + FOLFOX/FOLFIRI Cetuximab + Avastin + FOLFOX/FOLFIRI EORTC QLQ-C30 Global health/quality of life p=0.164 Physical functioning p=0.22 Role functioning p=0.263 Social functioning p=0.756 Emotional functioning p=0.769 Cognitive functioning p=0.785 Dermatology-specific QoL (DSQL) Skin symptoms p<0.001 Limitations in social activities due to skin condition p=0.008 Concern about appearance p<0.0001 • Patients randomised to Avastin + FOLFOX/FOLFIRI reported fewer skin symptoms and fewer social limitations and appearance concerns than patients receiving cetuximab alone or cetuximab + Avastin • Results were independent of chemotherapy partner (FOLFOX or FOLFIRI) • Global QoL and major QoL domains (physical, role, social and emotional functioning) were not significantly different across treatment arms Naughton, et al. ASCO 2013
- 30. SAKK 41/06
- 31. SAKK 41/06: non-inferiority trial of Avastin continuation vs no continuation after 1L Avastin + CT Previously untreated mCRC (n=262) Continued Avastin (n=131) R No treatment (n=131) • Phase III • Primary endpoint: non-inferiority in TTP (from randomisation) • Secondary endpoints: PFS, time to second-line treatment, OS, adverse events related to Avastin, treatment costs Avastin + chemotherapy (4–6 months) Koeberle, et al. ASCO 2013 PD PD
- 32. SAKK 41/06: TTP (from randomisation) was numerically increased with continued Avastin vs no Avastin Patients at risk Avastin 131 40 14 8 6 5 3 2 1 No Avastin 131 22 10 7 5 1 1 1 0 TTPestimate 1.0 0.8 0.40 0.20 0 Time (months) 0 6 12 18 24 30 36 42 48 0.60 Continued Avastin No Avastin No. of events 124 123 Median (95% CI) 4.1 (3.1–5.4) 2.9 (2.8–3.8) HR 95% CI 0.74 (5.7–0.95) Non-inferiority p=0.47 Koeberle, et al. ASCO 2013 2.9 4.1
- 33. SAKK 41/06: increased TTP with continued Avastin vs no Avastin across subgroups 0.5 0.727 1.0 1.5 Favours Avastin Favours no Avastin All Age >59 Age >59 Female Male WHO 0 WHO 1 First-line OD/PR First-line SD First-line dur 19–20 First-line dur 21–24 First-line iri + fluo First-line oxa + fluo First-line fluo mono 1 organ >1 organ Koeberle, et al. ASCO 2013 Hazard ratio (95% Cl)
- 34. SAKK 41/06: PFS (from start of first-line therapy) significantly increased with continued Avastin vs no Avastin Avastin No Avastin Events, n 126 124 Median PFS, months 9.5 8.5 HR (95% CI) p value 0.75 (0.58‒0.96) 0.021 0 0.2 0.4 0.8 1.0 0.6 PFSestimate 131 122 40 13 6 6 5 3 2 1 131 116 18 8 7 4 1 1 0 0 Avastin No Avastin Pts at risk 0 6 12 18 24 30 36 42 48 54 60 Time (months) 8.5 9.5 Koeberle, et al. ASCO 2013
- 35. SAKK 41/06: OS (from start of first line therapy) numerically increased with continued Avastin vs no Avastin Avastin No Avastin Events, n 84 84 Median OS, months 25.1 22.8 HR (95% CI) p value 0.83 (0.61‒1.12) 0.218 131 130 115 86 52 33 22 10 3 1 131 131 107 76 44 25 13 6 1 1 Avastin No Avastin No. at risk 1 0 0 6 12 18 24 30 36 42 48 54 60 Time (months) 64 0 0.2 0.4 0.8 1.0 0.6 OSestimate 22.8 25.1 Koeberle, et al. ASCO 2013
- 36. SAKK 41/06: safety Adverse event, % Avastin (n=131) No Avastin (n=131) Grade 1–2 3–4 5 1–2 3–4 5 Haemorrhage 5 – – 1 – – Hypertension 15 6 – 3 1 – Proteinuria 15 – – 1 – – Thrombosis – 2 – – – – GI perforation – – – – – – Koeberle, et al. ASCO 2013 No new safety signals when continuing Avastin until first progression Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 37. EAGLE
- 38. EAGLE: 2L Avastin + FOLFIRI in patients with mCRC who have failed 1L Avastin + oxaliplatin-based therapy *Evaluated using RECIST criteria (version 1.1) Tamagawa, et al. ASCO 2013 • Phase III • Primary endpoint: PFS* • Secondary endpoints: safety, RR, TTF, OS, OS from the start of the IL treatment, PFS from start of 1L treatment Patients with mCRC previously treated with oxaliplatin-based CT + Avastin 5mg/kg (n=387) Avastin 5mg/kg + FOLFIRI (n=193) Avastin 10mg/kg + FOLFIRI (n=194) R Arm A Arm B PD PD
- 39. EAGLE: no significant difference in PFS between Avastin 5mg/kg and Avastin 10mg/kg, combined with FOLFIRI • No significant difference was seen between Avastin doses in PFS from start of 2L therapy (HR=1.00; p=0.976) 1.0 0.8 0.6 0.4 0.2 PFSestimate Tamagawa, et al. ASCO 2013 0 6 12 18 24 30 36 181 86 22 2 1 0 0 187 88 18 3 1 0 0 Time (months)Avastin 5mg/kg + FOLFIRI Avastin 10mg/kg + FOLFIRI 0 Avastin 5 mg/kg + FOLFIRI (arm A) (n=180) Avastin 10 mg/kg + FOLFIRI (arm B) (n=187) Median, months 6.1 6.4 HR (95% CI) p value 0.95 (0.75–1.21) p=0.676 6.1 6.4
- 40. Internal Use Only EAGLE: Avastin 5mg/kg and 10mg/kg combined with FOLFIRI show no significant difference in PFS from start of 1L therapy Tamagawa, et al. ASCO 2013 Avastin 5mg/kg + FOLFIRI (arm A) (n=180) Avastin 10mg/kg + FOLFIRI (arm B) (n=187) Median, months 17.4 17.6 HR (95% CI) p value 1.00 (0.79–1.26) 0.976 Avastin 5mg/kg 181 176 137 76 42 18 6 3 2 1 0 10mg/kg 186 183 146 72 40 18 12 5 3 1 0 1.0 0.6 0.2 0 PFSestimate 0 6 12 18 24 30 36 42 48 54 60 0.8 0.4 Time (months) 17.4 17.6
- 41. NE = not evaluable Tamagawa, et al. ASCO 2013 EAGLE: no significant difference in 2L response between Avastin 5mg/kg and Avastin 10mg/kg combined with FOLFIRI Avastin 5 mg/kg + FOLFIRI (n=180) Avastin 10 mg/kg + FOLFIRI (n=187) Partial response, % 11.1 10.7 p=1.00 Stable disease, % 70.6 70.6 Disease progression, % 13.9 11.8 Not evaluable, % 4.4 7.0
- 42. EAGLE: toxicity in both arms was consistent with previously reported studies and showed no increase with higher doses of Avastin All (n=365) Avastin 5mg/kg + FOLFIRI (n=180) Avastin 10mg/kg + FOLFIRI (n=185) Adverse event, % Grade ≥3 Any grade Grade ≥3 Any grade Grade ≥3 Any grade WBC 16.2 64.1 15.0 67.2 17.3 61.1 Neutropenia 44.9 64.9 48.3 67.0 41.6 63.2 Haemoglobin 3.0 64.7 2.2 66.5 3.8 63.2 PLT 0.8 35.1 0.6 34.6 1.1 35.7 T-Bill 0.0 7.7 0.0 6.7 0.0 8.6 AST 0.8 40.3 0.0 43.6 1.6 37.3 ALT 0.5 25.2 0.0 25.1 1.1 25.4 ALP 0.3 49.3 0.6 52.5 0.0 46.5 Fatigue 9.9 60.3 8.3 58.7 11.4 62.2 Anorexia 5.8 62.7 6.1 62.6 5.4 63.2 Nausea 4.7 51.0 5.0 50.8 4.3 51.4 Vomiting 3.3 22.2 2.8 19.6 3.8 24.9 Diarrhoea 2.5 41.4 3.3 39.7 1.6 43.2 Stomatitis 3.3 48.5 2.8 49.7 3.8 47.6 Alopecia 0.0 41.1 0.0 39.7 0.0 42.7 Tamagawa, et al. ASCO 2013 Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 43. EAGLE: toxicity in both arms was consistent with previously reported studies and showed no increase with higher doses of Avastin All (n=365) Avastin 5mg/kg + FOLFIRI (n=180) Avastin 10mg/kg + FOLFIRI (n=185) Adverse event, % Grade ≥3 Any grade Grade ≥3 Any grade Grade ≥3 Any grade Hypertension 1.1 16.7 1.1 14.5 1.1 18.9 Proteinurea 0.5 38.1 1.1 39.7 0.0 36.8 Constipation 0.0 7.7 0.0 5.6 0.0 9.7 Neuropathy 3.8 58.1 3.9 57.5 3.8 58.9 GI haemorrhage 0.3 4.1 0.6 5.0 0.0 3.2 Epistaxis 0.3 19.2 0.0 16.2 0.5 22.2 Arterial thrombosis 0.8 0.8 0.6 0.6 1.1 1.1 Venous thrombosis 1.1 1.4 1.1 1.1 1.1 1.6 GI perforation 0.5 0.5 0.6 0.6 0.5 0.5 Treatment-related death 1.1 1.1 1.1 Tamagawa, et al. ASCO 2013
- 44. TRIBE
- 45. TRIBE: 1L Avastin + FOLFOXIRI vs Avastin + FOLFIRI followed by Avastin until progression Loupakis, et al. ASCO GI 2013 Previously untreated, unresectable mCRC (n=508) Avastin + FOLFOXIRI (up to 12 cycles) Avastin + FOLFIRI (up to 12 cycles) R Avastin + 5-FU/LV Avastin + 5-FU/LV PD PD Induction Maintenance • Phase III • Primary endpoint: PFS • Secondary endpoints: response rate, secondary R0 resection rate, OS, safety, biomarker evaluation
- 46. 1.0 0.6 0.2 0 PFSestimate 0 6 12 18 24 30 36 42 48 54 0.8 0.4 Time (months) 9.7 12.1 FOLFIRI + Avastin (n=256) FOLFOXIRI + Avastin (n=252) Progressed, n 213 226 Median PFS, months 9.7 12.1 Unstratified HR (95% CI) p value 0.77 (0.64‒0.93) 0.006 Stratified HR (95% CI) p value 0.75 (0.62–0.80) 0.003 TRIBE: 1L FOLFOXIRI + Avastin produces superior PFS to FOLFIRI + Avastin Falcone, et al. ASCO 2013
- 47. TRIBE: Avastin + FOLFOXIRI improved PFS vs Avastin + FOLFIRI in all subgroups except those treated with adjuvant therapy Factor n HR p Adjuvant treatment No 444 0.7 0.039 Yes 64 1.3 Performance status 0 456 0.79 0.2 1–2 52 0.53 Site of primary Left 330 0.82 0.288 Right 149 0.66 Liver only disease No 402 0.74 0.293 Yes 105 0.95 Type of metastases Metachronous 103 0.92 0.356 Synchronous 404 0.73 Resection of primary No 166 0.77 0.997 Yes 341 0.77 Kohne score High 47 0.83 0.822 Intermediate 224 0.72 Low 213 0.81 Experimental better Control better 0.5 1 1.5 2 Falcone, et al. ASCO 2013
- 48. TRIBE: Avastin + FOLFOXIRI improved PFS vs Avastin + FOLFIRI in all subgroups analysed by KRAS or BRAF status Experimental better Control better Factor n HR p KRAS status MT 200 0.84 0.973 WT 193 0.83 BRAF status MT 28 0.55 0.323 WT 365 0.83 0.4 0.6 0.8 1 Falcone, et al. ASCO 2013
- 49. Avastin + FOLFIRI (n=256) Avastin + FOLFOXIRI (n=252) p value Overall response rate (%) 53 65 0.006 Complete response (%) 3 5 Partial response (%) 50 60 Stable disease (%) 32 25 Progressive disease (%) 11 6 Not assessed (%) 4 4 Secondary surgery with radical intent (%) 21 26 0.210 R0 secondary surgery (%) 12 15 0.327 Liver-only subgroup (n=46) (n=59) Secondary surgery with radical intent (%) 41 39 1.000 R0 secondary surgery (%) 28 32 0.823 TRIBE: significant increase in response rate but not R0 resection rate with Avastin + FOLFOXIRI Falcone, et al. ASCO 2013
- 50. 1.0 0.6 0.2 0 Time (months) OSestimate 0 6 12 18 24 30 36 42 48 54 0.8 0.4 Patients at risk: FOLFIRI + Avastin 256 233 216 172 109 69 36 15 5 0 FOLFIRI + Avastin 252 234 205 175 119 70 35 15 4 0 FOLFIRI + Avastin FOLFOXIRI + Avastin Median OS, mos 25.8 31.0 Unstratified HR (95% CI) p value 0.83 (0.66–1.05) 0.125 Stratified HR (95% CI) p value 0.79 (0.63–1.00) 0.054 25.8 31.0 TRIBE: trend towards improved OS with Avastin + FOLFOXIRI (data immature) Median follow-up 32.3 months Falcone, et al. ASCO 2013
- 51. TRIBE: toxicity profile – safety population Grade 3/4 adverse events (%) FOLFIRI + Avastin (n=254) FOLFOXIRI + Avastin (n=250) p value Nausea 3 3 1.000 Vomiting 3 4 0.492 Diarrhoea 11 19 0.012 Stomatitis 4 9 0.048 Neutropenia 20 50 <0.001 Febrile neutropenia 6 9 0.315 Neurotoxicity 0 5 <0.001 Hypertension 2 5 0.157 Venous thrombosis 6 7 0.593 Arterial thrombosis 2 1 1.000 Bleeding 1 1 1.000 Falcone, et al. ASCO 2013 Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 53. Previously untreated, KRAS exon 2 WT mCRC (n=285) Panitumumab + mFOLFOX6 (n=142) Avastin + mFOLFOX6 (n=143) R • Primary endpoint: PFS • Secondary endpoints: OS, ORR, resection rate, safety, treatment effect in WT RAS tumours and WT RAS/BRAF tumours • Patients with disease WT for KRAS exon 2 may have mutations in KRAS exons 3 or 4 or NRAS exons 2, 3 or 4. This retrospective analysis compared outcomes in these patients vs patients with disease WT in KRAS and NRAS (exons 2, 3 and 4) [no activating mutations] • ‘Gold standard’ bidirectional Sanger sequencing and WAVE-based SURVEYOR scan kits were used to detect mutations in KRAS exon 3, exon 4; NRAS exon 2, exon 3, exon 4; and BRAF exon 15 • An additional analysis was performed ~1 year after the last patient was enrolled: primary analysis data cutoff was 30 May 2012; additional OS analysis data cutoff was 3 January 2013 PEAK: phase II retrospective analysis of efficacy by KRAS/NRAS mutation status Schwartzberg , et al. ASCO 2013
- 54. PEAK: RAS mutations outside KRAS exon 2 and in NRAS are associated with resistance to EGFR inhibition Outcome Panitumumab + FOLFOX6 Avastin + FOLFOX6 HR (95% CI) Data cut-off May 2012 KRAS exon 2 WT PFS 10.9 10.1 0.87 (0.65‒1.17); p=0.35 KRAS WT/ RAS WT* PFS 13.0 9.5 0.65 (0.44‒0.96); p=0.03 KRAS exon 2 WT OS NR 25.4 0.72 (0.47‒1.11); p=0.14 KRAS WT/ RAS WT* OS NR 29.0 0.61 (0.34‒1.09); p=0.09 KRAS WT/ other RAS MT‡ PFS 7.8 8.9 1.39 (0.73‒2.64); p=0.32 Data cut-off January 2013 KRAS exon 2 WT PFS 10.9 10.1 0.84 (0.64‒1.11); p=0.22 KRAS WT/ RAS WT* PFS 13.0 10.1 0.66 (0.46‒0.95); p=0.03 KRAS exon 2 WT OS 34.2 24.3 0.62 (0.44‒0.89); p=0.009 KRAS WT/ RAS WT* OS 41.3 28.9 0.63 (0.39‒1.02); p=0.058 NR = not reached * WT in exons 2, 3 and 4 of KRAS/NRAS ‡ WT KRAS (exon 2) and MT NRAS (exons 2, 3 or 4) Data cut-off 3 January 2013 Schwartzberg , et al. ASCO 2013
- 55. PEAK KRAS/NRAS analysis – improved PFS in patients with WT RAS mCRC treated with panitumumab + mFOLFOX Panitumumab +mFOLFOX6 (n=142) Avastin + mFOLFOX6 (n=143) Events, n/N (%) 100/142 (70) 108/143 (76) Median, months 10.9 10.1 Stratified HR (95% CI) p value 0.84 (0.64‒1.11) 0.22 Panitumumab +mFOLFOX6 (n=88) Avastin + mFOLFOX6 (n=82) Events, n/N (%) 57/88 (65) 66/82 (80) Median, months 13.0 10.1 Stratified HR (95% CI) p value 0.66 (0.46‒0.95) 0.03 0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8 1.0 Time (months) Time (months) PFSestimate PFSestimate WT KRAS exon 2 (ITT set) WT RAS (exons 2, 3, 4 of KRAS/NRAS) 10.1 10.9 10.1 13.0 Schwartzberg , et al. ASCO 2013; data cut-off 3 January 2013 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 • Decreased PFS was observed in patients with MT RAS tumours in the panitumumab + FOLFOX6 arm compared to the Avastin + FOLFOX6 arm
- 56. Internal Use Only 0 2 4 6 8 10 12 14 16 18 20 PEAK KRAS/NRAS analysis – no improvement in PFS with panitumumab + mFOLFOX compared to Avastin + mFOLFOX6 in patients with KRAS exon 2 WT/RAS MT mCRC Data cut-off 30 May 2013 Schwartzberg, et al. ASCO 2013 Panitumumab + mFOLFOX6 (n=24) Avastin + mFOLFOX6 (n=27) Events, n/N (%) 21/24 (88) 18/27 (67) Median, months (95% CI) 7.8 (6.5‒9.8) 8.9 (7.3‒12.0) Stratified HR (95% CI) p value 1.39 (0.73‒2.64) 0.32 1.0 0.6 0.2 0 PFSestimate 0.8 0.4 Time (months) 7.8 8.9
- 57. PEAK KRAS/NRAS analysis – OS in pts with WT RAS exon 2 and WT RAS mCRC treated with panitumumab + mFOLFOX6 Panitumumab +mFOLFOX6 (n=142) Avastin + mFOLFOX6 (n=143) Events, n/N (%) 52/142 (37) 78/143 (55) Median, months 34.2 24.3 Stratified HR (95% CI) p value 0.62 (0.44‒0.89) 0.009 WT KRAS exon 2 (ITT set) WT RAS (exons 2, 3, 4 of KRAS/NRAS) 0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8 1.0 Time (months) Time (months) OSestimate OSestimate 24.3 34.2 28.9 41.3 Schwartzberg , et al. ASCO 2013; data cut-off 3 January 2013 Panitumumab +mFOLFOX6 (n=88) Avastin + mFOLFOX6 (n=82) Events, n/N (%) 30/88 (34) 40/82 (49) Median, months 41.3 28.9 Stratified HR (95% CI) p value 0.63 (0.39‒1.02) 0.058 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 3234 36 3840 42 44 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42
- 58. PEAK: ORR and safety *WT in exons 2, 3 and 4 of KRAS/NRAS ǂWT KRAS (exon 2) and MT KRAS (exons 3 or 4) or MT NRAS (exons 2, 3 or 4) Data cut-off 30 May 2012 Schwartzberg , et al. ASCO 2013 Panitumumab + FOLFOX6 Avastin + FOLFOX6 Primary analysis (n=142) (n=142) ORR, % 58 54 WT RAS* (n=88) (n=81) ORR, n (%) 64 60 WT KRAS-2, MT RASǂ (n=24) (n=27) Median OS (months) 58 56 • The safety profile in both treatment arms was similar to previously reported studies and treatment discontinuation rates due to adverse events were similar between treatment arms • Biomarker subpopulations showed similar adverse event rates compared with the primary analysis population Schwartzberg , et al. ASCO 2013
- 60. PRIME: retrospective analysis of efficacy by KRAS/NRAS mutation status Previously untreated mCRC (n=1,183) (n=641 in KRAS/NRAS analysis) Panitumumab + FOLFOX4 (n=593) (n=325 KRAS WT (exon 2); n=320 in KRAS/NRAS analysis) FOLFOX4 (n=590) (n=331 KRAS WT (exon 2); n=321 in KRAS/NRAS analysis) • Phase III • Primary endpoint: PFS • Secondary endpoints: OS, ORR, TTP, DOR, safety • Sponsor: Amgen • PRIME was amended prior to efficacy analysis and completion of enrolment to focus on hypothesis testing in the KRAS WT subset: the primary objective was to evaluate treatment effect on PFS and OS in pts WT for RAS (WT for KRAS and NRAS exons 2, 3 and 4) or WT for RAS and BRAF (WT for KRAS and NRAS exons 2, 3 and 4 and BRAF exon 15) • Interaction texts were conducted to compare panitumumab treatment effect between WT RAS and MT RAS or WT RAS and WT KRAS exon 2/MT other RAS to assess the predictive value for RAS • A new testing method was used (‘gold standard’ bidirectional Sanger sequencing and WAVE-based SURVEYOR scan kits) to detect mutations in KRAS exon 3, exon 4; NRAS exon 2, exon 3, exon 4; and BRAF exon 15 R Oliner, et al. ASCO 2013
- 61. PRIME: panitumumab + FOLFOX4 has a detrimental effect in patients with RAS MT mCRC Panitumumab + FOLFOX4 (n=320) FOLFOX4 (n=321) HR; p value WT KRAS exon 2 [original test] Median OS (months) 23.9 19.7 0.83; 0.072 Median PFS (months) 9.6 8.0 0.80; 0.02 WT KRAS exon 2/MT other RAS [new test] Median OS (months) 17.1 18.3 1.29; 0.305 Median PFS (months) 7.3 8.0 1.28; 0.326 WT RAS [new test] Median OS (months) 26.0 20.2 0.78; 0.043 Median PFS (months) 10.1 7.9 0.72; 0.004 MT RAS [new test] Median OS (months) 15.6 19.2 1.25; 0.034 Median PFS (months) 7.3 8.7 1.31; 0.008 • The addition of panitumumab to FOLFOX4 produced – significantly increased PFS and OS in patients with RAS WT mCRC – significantly detrimental effect on PFS and OS in patients with RAS MT mCRC Oliner, et al. ASCO 2013
- 62. PRIME: poorer PFS with panitumumab + FOLFOX4 in mCRC WT for KRAS exon 2 but MT for other RAS exons WT KRAS exon 2 (original KRAS WT testing) WT KRAS exon 2/MT other RAS (new testing) 8.0 9.6 Time (months) PFSestimate 7.3 8.0 PFSestimate Time (months) 0 2 4 6 8 10 12 14 16 18 20 22 24 0 2 4 6 8 10 12 14 16 18 20 22 Panitumumab + FOLFOX4 FOLFOX4 alone Events, n 199/325 (61) 215/331 (65) Median PFS, months 9.6 (9.2–11.1) 8.0 (7.5–9.3) HR (95% CI) p value 0.80 (0.56‒0.97) 0.02 Panitumumab + FOLFOX4 FOLFOX4 alone Events, n 38/51 (75) 35/57 (61) Median PFS, months 7.3 (5.3–9.2) 8.0 (6.4–11.3) HR (95% CI) p value 1.28 (0.79‒2.07) 0.326 Oliner, et al. ASCO 2013 1.0 0.8 0.6 0.4 0.2 0.0 1.0 0.8 0.6 0.4 0.2 0.0
- 63. PRIME: poorer OS with panitumumab + FOLFOX4 in mCRC KRAS exon 2 WT but MT for other RAS exons Oliner, et al. ASCO 2013 1.0 0.8 0.6 0.4 0.2 0.0 0 6 12 14 24 30 32 34 3642 8 10 16 18 2620 28 0 6 12 14 24 30 32 3442 8 10 16 18 2620 2822 22 1.0 0.8 0.6 0.4 0.2 0.0 Panitumumab + FOLFOX4 FOLFOX4 alone Events, n 185/325 (51) 190/331 (57) Median OS, months 23.9 (20.3–28.2) 19.7 (17.6–22.6) HR (95% CI) p value 0.83 (0.67‒1.02) 0.072 Panitumumab + FOLFOX4 FOLFOX4 alone Events, n 35/51 (69) 33/57 (58) Median OS, months 17.1 (10.8–19.4) 18.3 (13.0–23.2) HR (95% CI) p value 1.29 (0.79‒2.10) 0.305 19.7 23.9 18.317.1 WT KRAS exon 2 (original KRAS WT testing) WT KRAS exon 2/MT other RAS (new testing) Time (months) OSestimate OSestimate Time (months)
- 64. PRIME: mutations in BRAF do not appear to be predictive for panitumumab treatment effect Oliner, et al. ASCO 2013 Panit + FOLFOX4 (n=320) FOLFOX4 (n=321) HR; p value WT RAS/WT BRAF Median OS (months) 28.3 20.9 0.74; 0.023 Median PFS (months) 10.8 9.2 0.68; 0.002 WT RAS/MT BRAF Median OS (months) 10.5 9.2 0.90; 0.764 Median PFS (months) 6.1 5.4 0.58; 0.116 MT RAS or MT BRAF Median OS (months) 15.3 18.0 1.21; 0.064 Median PFS (months) 7.3 8.0 1.24; 0.027 WT KRAS exon 2/MT other RAS or MT BRAF Median OS (months) 14.5 15.8 1.14; 0.508 Median PFS (months) 6.7 7.3 1.05; 0.797
- 65. PRIME: adverse events in panitumumab arm similar to those reported for patients with KRAS exon 2 WT mCRC Oliner, et al. ASCO 2013 WT RAS MT RAS % Panit + FOLFOX4 (n=256) FOLFOX4 alone (n=250) Total (n=506) Panit + FOLFOX4 (n=268) FOLFOX4 alone (n=275) Total (n=543) Any adverse event 100 99 100 99 99 99 Worst grade of 3 57 50 53 57 53 55 Worst grade of 4 28 20 24 24 20 22 Worst grade of 5 5 6 6 7 4 5 Any serious adverse event 43 37 40 45 31 38 Leading to permanent discontinuation of any study drug 25 16 21 22 13 18 Not serious 19 11 15 19 9 14 Serious 9 6 8 6 5 6
- 67. Patients with previously untreated mCRC (n=700) Avastin* + mFOLFOX7 RAvastin*+ XELOX2 Avastin* + FOLFIRI NOPROGRESSION Avastinǂ + erlotinib (n=224) Avastinǂ (n=228) PD PD DREAM: maintenance Avastin with or without erlotinib in mCRC, by KRAS status *5 mg/kg q2w; ǂ7.5 mg/kg q3w Tournigand, et al. ASCO 2013 • Phase III • Primary endpoint: PFS on maintenance therapy • Secondary endpoints include: OS, survival according to KRAS status Induction (n=700) Maintenance (n=452) REGISTRATION
- 68. DREAM: PFS (from randomisation) significantly increased with maintenance Avastin + erlotinib vs Avastin alone (ITT population) Avastin (n=228) Avastin + erlotinib (n=224) Median PFS, months 4.6 5.9 HR (95% CI) p value 0.7 (0.61‒0.94) 0.0096 Patients at risk Avastin 228 179 115 74 42 29 17 13 10 7 3 2 1 Avastin + erlotinib 224 182 124 82 58 39 30 20 17 12 7 4 2 1.0 0.6 0.2 0 PFSestimate 0 2 4 6 8 10 12 14 16 18 20 22 24 0.8 0.4 Time (months) 4.6 5.9 Tournigand, et al. ASCO 2013
- 69. DREAM: similar OS (from registration) with maintenance Avastin + erlotinib vs Avastin alone (ITT population) Avastin (n=228) Avastin + erlotinib (n=224) Median OS, months 27.9 28.4 HR (95% CI) p value 0.89 (0.70‒1.12) 0.8857 Patients at risk Avastin 228 224 193 143 99 77 46 25 10 3 0 Avastin + erlotinib 224 220 187 140 99 73 43 31 20 11 4 1.0 0.6 0.2 0 PFSestimate 0 6 12 18 24 30 36 42 48 54 60 0.8 0.4 Time (months) 27.9 28.4 Tournigand, et al. ASCO 2013
- 70. DREAM: similar PFS (from randomisation) with maintenance Avastin + erlotinib vs Avastin alone in KRAS WT mCRC Avastin Avastin + erlotinib WT KRAS, n 111 129 Median PFS, months 5.9 6.0 HR (95% CI) p value 0.86 (0.64‒1.16) 0.135 Patients at risk Avastin 111 91 60 42 25 19 11 9 7 5 3 2 1 Avastin + erlotinib 129 105 74 51 35 23 18 13 11 7 4 3 2 1.0 0.6 0.2 0 PFSestimate 0 2 4 6 8 10 12 14 16 18 20 22 24 0.8 0.4 Time (months) 5.9 6.0 Tournigand, et al. ASCO 2013
- 71. Avastin Avastin + erlotinib MT KRAS, n 95 78 Median PFS, months 4.4 4.7 HR (95% CI) p value 0.77 (0.54‒1.08) 0.124 Patients at risk Avastin 95 76 47 27 17 10 6 4 3 2 0 0 0 Avastin + erlotinib 78 61 38 25 18 11 8 6 6 5 3 1 0 1.0 0.6 0.2 0 PFSestimate 0 2 4 6 8 10 12 14 16 18 20 22 24 0.8 0.4 Time (months) 4.4 4.7 Tournigand, et al. ASCO 2013 DREAM: similar PFS (from randomisation) with maintenance Avastin + erlotinib vs Avastin alone in KRAS MT mCRC
- 72. DREAM: toxicity of Avastin was consistent with the reported safety profile Tournigand, et al. ASCO 2013 Grade 3/4 adverse events, % Avastin (n=228) Avastin + erlotinib (n=224) Vomiting 0 1 Diarrhoea 1 9 Proteinuria <1 <1 Hypertension 3 3 Skin toxicity 0 20 Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 74. • Phase III • Primary endpoint: PFS • Secondary endpoints: OS, response rate, safety • Patients had a median age of 76 (70‒87) years and were recruited in 10 countries • This was a post-hoc analysis of PFS, OS and safety in patients grouped according to age: 70–74 years, 75–79 years and ≥80 years Patients aged ≥70 years with previously untreated mCRC (n=280) Capecitabine Avastin + capecitabine R PD PD AVEX age subgroup analysis: Avastin + capecitabine vs capecitabine for the 1L treatment of elderly mCRC patients Saunders, et al. ASCO 2013
- 75. AVEX age subgroup analysis: PFS significantly improved with Avastin + capecitabine across age subgroups Saunders, et al. ASCO 2013 70–74 years 75–79 years ≥80 years Outcome Avastin + cape (n=55) Cape (n=46) Avastin + cape (n=57) Cape (n=66) Avastin + cape (n=28) Cape (n=28) PFS Median, months 7.6 5.0 9.8 5.1 10.5 5.1 HR (95% CI) 0.52 (0.32‒0.83) 0.60 (0.40‒0.89) 0.36 (0.19‒0.71) p value <0.001 0.016 0.003 OS Median, months 20.7 22.2 19.8 17.4 19.7 12.6 HR (95% CI) 0.91 (0.50‒1.66) 0.79 (0.48‒1.30) 0.62 (0.31‒1.24) p value 0.55 0.37 0.24 • PFS was significantly improved with the addition of Avastin to capecitabine in all age subgroups analysed • Differences in OS between treatment arms were not significant in any of the age subgroups evaluated
- 76. Saunders, et al. ASCO 2013 AVEX age subgroup analysis: ORR numerically improved with Avastin + capecitabine across age subgroups 70–74 years 75–79 years ≥80 years Outcome, % Avastin + cape (n=55) Cape (n=46) Avastin + cape (n=57) Cape (n=66) Avastin + cape (n=28) Cape (n=28) ORR 25.5 10.9 15.8 12.1 14.3 3.6 p=0.076 p=0.607 p=0.352 CR 3.6 0 1.8 1.5 0 3.6 PR 21.8 10.9 14.0 10.6 14.3 0 SD 49.1 56.5 61.4 43.9 53.6 42.9 PD 12.7 17.4 8.8 21.2 7.1 28.6 DCR 74.5 67.4 77.2 56.1 67.9 46.4 • ORR and DCR were numerically improved in the Avastin + capecitabine arms in each of the three age subgroups • In the primary analysis of AVEX (median patient age 76 (70‒87) years) ORR was significantly increased with Avastin + capecitabine compared to capecitabine alone (19.3% vs 10.0%; p=0.042)
- 77. • Grade ≥3 AEs were more common in the Avastin + capecitabine arm across age subgroups • Patients aged 70–74 years receiving Avastin + capecitabine had a higher incidence of serious AEs than those receiving capecitabine Saunders, et al. ASCO 2013 AVEX age subgroup analysis: AEs of special interest to Avastin occurred at similar rates in each age subgroup 70–74 years 75–79 years ≥80 years Grade ≥3 adverse events, % Avastin + cape (n=54) Cape (n=46) Avastin + cape (n=53) Cape (n=64) Avastin + cape (n=27) Cape (n=26) Bleeding/haemorrhage – 2.2 – – – – Hypertension 5.6 2.2 – 1.6 – – VTE 9.3 6.5 11.3 4.7 – – Proteinuria 1.9 – 1.9 – – – ATE 1.9 – 1.9 – 11.1 7.7 Wound-healing complications – – – – – – Pulmonary haemorrhage/ haemoptysis – – – – – 3.8 CHF – 2.2 – – – – Fistulae – – – – – – GI perforation – – – – – – RPLS – – – – – – Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 78. Saunders, et al. ASCO 2013 70–74 years 75–79 years ≥80 years Grade ≥3 adverse events, % Avastin + cape (n=54) Cape (n=46) Avastin + cape (n=53) Cape (n=64) Avastin + cape (n=27) Cape (n=26) Hand-foot syndrome 16.7 6.5 13.2 9.4 14.8 – Diarrhoea 3.7 4.3 7.5 4.7 11.1 15.4 Asthenia 1.9 – 5.7 6.3 11.1 7.7 Fatigue 3.7 – 3.8 – 3.7 3.8 Nausea – – – – 3.7 – Vomiting – – 1.9 – 3.7 7.7 Stomatitis – – – – – 3.8 Neutropenia – – – – 3.7 3.8 AVEX age subgroup analysis: AEs of special interest to chemotherapy occurred at similar rates in each age subgroup Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 79. OLIVIA
- 80. • Phase II • Primary endpoint: overall resection rate (R0/R1/R2) • Secondary endpoints: ORR (by RECIST), PFS, RFS, OS and safety OLIVIA: phase II study of Avastin + mFOLFOX6 vs Avastin + FOLFOXIRI in initially unresectable liver-limited mCRC mCRC patients with liver-only metastases defined by an MDT as unresectable (n=80) Avastin + mFOLFOX6 (up to 12 cycles) (n=39) Avastin + FOLFOXIRI (up to 12 cycles) (n=41) R Gruenberger, et al. ASCO 2013
- 81. OLIVIA: Avastin + FOLFOXIRI is associated with higher resection and response rates than Avastin + mFOLFOX6 in mCRC (ITT population) Gruenberger, et al. ASCO 2013 Avastin + FOLFOXIRI (n=41) Avastin + mFOLFOX6 (n=39) Difference (95% CI) p value Resection rate, % R0/R1/R2 61.0 48.7 12.3 (‒11.0–35.5) 0.271 R0/R1 51.2 33.3 17.9 (‒5.0–40.7) 0.106 R0 48.8 23.1 25.7 (3.9–47.5) 0.017 ORR, % 80.5 61.5 18.9 (‒2.1–40.0) 0.061 Median PFS, months 18.8 12.0 – 0.0002 • The primary endpoint of overall resection rate (R0/R1/R2) was numerically higher with Avastin + FOLFOXIRI than with Avastin + FOLFOX6 • R0 resection rate and median PFS were significantly higher and ORR was numerically higher with Avastin + FOLFOXIRI than with Avastin + mFOLFOX6
- 82. 1.0 0.6 0.2 0 PFSestimate 0 4 8 12 16 20 24 28 32 0.8 0.4 Pts at risk: 39 37 26 16 5 41 38 35 27 21 2 1 0 0 12 4 2 0 12.0 18.8 Time (months) Gruenberger, et al. ASCO 2013 OLIVIA: prolonged PFS in Avastin + FOLFOXIRI vs Avastin + mFOLFOX6 in 1L mCRC (ITT population) mFOLFOX6 + Avastin FOLFOXIRI + Avastin Median, months 12.0 18.8 p-value p=0.0002
- 83. OLIVIA: no new safety concerns Gruenberger, et al. ASCO 2013 Grade 3–5 adverse event, % Avastin + FOLFOXIRI (n=40) Avastin + mFOLFOX6 (n=37) Any adverse event 95.0 83.8 Neutropenia 47.5 35.1 Febrile neutropenia 12.5 8.1 Diarrhoea 27.5 13.5 Vomiting 7.5 2.7 Fatigue 7.5 2.7 Wound dehiscence 7.5 0 Pulmonary embolism 2.5 5.4 Deep vein thrombosis 5.0 5.4 Constipation 0 5.4 Gamma-glutamyltransferase increased 0 5.4 • The most common grade 3–5 haematological adverse event was neutropenia • The most common non-haematological adverse events were diarrhoea and vomiting Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
- 84. Ml18147 (TML) – patterns of disease progression and outcomes based on extent of disease
- 85. ML18147 (TML): phase III trial comparing Avastin + chemotherapy beyond first progression vs chemotherapy Avastin + 1L doublet CT (n=820) Avastin + 2L doublet CT (n=409) 2L doublet CT (n=411) R • Phase III • Primary endpoint: OS from randomisation • Secondary endpoints: PFS from randomisation, best ORR, safety PD PD Greil, et al. ASCO 2013
- 86. 64 50 6 14 4 2 19 67 42 7 12 4 2 23 80 60 40 20 10 0 Site of metastasis Patients(%) Lung Liver Peritoneum Lymph Bone Pleura Other nodes 70 50 30 Chemotherapy Avastin + chemotherapy ML18147: similar patterns of PD in patients treated with Avastin + CT vs CT beyond progression (all progressions) Greil, et al. ASCO 2013
- 87. 50 40 30 20 10 0 Site of metastasis Patients(%) Lung Liver Peritoneum Lymph Bone Pleura Other nodes 45 33 6 10 6 2 17 39 42 10 8 4 2 14 ML18147: similar patterns of PD in patients treated with Avastin + CT vs CT beyond progression (PD due to new lesions) Chemotherapy Avastin + chemotherapy Greil, et al. ASCO 2013
- 88. CT 117 82 46 13 5 2 2 1 0 Av + CT 109 91 52 14 5 2 1 0 0 9.3 11.6 HR (95% CI)=0.79 (0.59–1.06) 1.0 0.8 0.6 0.4 0.2 0.0 Time (months)OSestimate 0 6 12 18 24 30 36 42 48 10.0 11.0 HR (95% CI)=0.81 (0.67–0.97) Liver-limited disease Non-liver-limited disease Pts at risk: ML18147: OS was similar in patients with liver-limited or non- liver-limited disease at baseline Chemotherapy Avastin + chemotherapy Chemotherapy Avastin + chemotherapy CT 292 210 115 38 19 5 1 1 0 Av + CT 300 237 136 50 24 11 3 1 0 1.0 0.8 0.6 0.4 0.2 0.0 0 6 12 18 24 30 36 42 48 OSestimate Time (months) Greil, et al. ASCO 2013
- 89. 1.0 0.8 0.6 0.4 0.2 0.0 0 6 12 18 24 30 36 42 48 4.1 5.7 4.1 5.6 ML18147: PFS was similar in patients with liver-limited and non-liver-limited disease at baseline Liver-limited disease Non-liver-limited disease CT 117 33 5 2 0 0 0 0 0 Av + CT 109 52 8 3 0 0 0 0 0 CT 292 86 15 4 4 0 0 0 0 Av + CT 300 137 37 9 5 2 0 0 0 PFSestimate Pts at risk: Time (months) HR (95% CI)=0.68 (0.52–0.89) HR (95% CI)=0.68 (0.57–0.80) Chemotherapy Avastin + chemotherapy Chemotherapy Avastin + chemotherapy 1.0 0.8 0.6 0.4 0.2 0.0 Time (months)PFSestimate 0 6 12 18 24 30 36 42 48 Greil, et al. ASCO 2013
- 90. SOFT
- 91. SOFT: phase III trial of 1L Avastin + mFOLFOX-6 vs Avastin + SOX • Phase III • Primary endpoint: non-inferiority in PFS • Secondary endpoints: OS, RR, disease control rate (DCR), TTP, time to treatment failure (TTF), resection rate (R0), safety Previously untreated mCRC patients (n=512) Avastin + mFOLFOX-6 Avastin + S-1/oxaliplatin (SOX) R Takahari, et al. ASCO 2013
- 92. Internal Use Only SOFT: PFS with 1L Avastin + S-1/oxaliplatin (SOX) was non-inferior to 1L Avastin + mFOLFOX6 0 6 12 18 24 30 36 42 1.0 0.6 0.2 0 PFSestimate 0.8 0.4 Time (months) 10.2 10.2 Takahari, et al. ASCO 2013 Avastin + mFOLFOX6 Avastin + SOX Median, months (95% CI) 10.2 (9.5‒11.3) 10.2 (9.4‒11.1) HR (95% CI) 1.021 (0.847‒1.232) • Response rate was 62.7% with Avastin + mFOLFOX6 vs 61.5% with Avastin + SOX
- 93. Internal Use Only 0 6 12 18 24 30 36 42 1.0 0.6 0.2 0 OSestimate 0.8 0.4 Time (months) SOFT: OS with 1L Avastin + S-1/oxaliplatin (SOX) and 1L Avastin + mFOLFOX6 was similar Avastin + mFOLFOX6 Avastin + SOX Median, months (95% CI) 30.9 (28.6‒33.1) 29.6 (25.8‒NR) HR (95% CI) 1.052 (0.805‒1.376) 29.6 30.9 Median follow-up duration: 23.4 (0.3‒37.8) months Takahari, et al. ASCO 2013
- 94. SOFT: no new safety concerns Grade 3–4 adverse event, % Avastin + mFOLFOX-6 Avastin + SOX Leucopenia 8.4 2.4 Neutropenia 33.7 8.8 Anorexia 1.2 5.2 Diarrhoea 2.8 9.2 Takahari, et al. ASCO 2013 Red box indicates a difference in incidence of grade ≥3 AE between treatment arms of ≥5%
