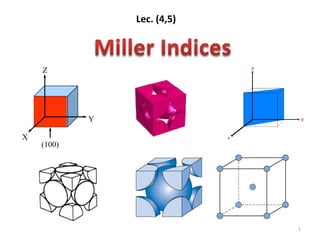
Miller indecies
- 2. 2 Crystal Systems 7 crystal systems 14 crystal lattices Unit cell: smallest repetitive volume that contains the complete lattice pattern of a crystal. a, b and c are the lattice constants
- 3. Unit Cells Types Primitive Face-Centered Body-Centered End-Centered A unit cell is the smallest component of the crystal that reproduces the whole crystal when stacked together. • Primitive (P) unit cells contain only a single lattice point. • Internal (I) unit cell contains an atom in the body center. • Face (F) unit cell contains atoms in the all faces of the planes composing the cell. • Centered (C) unit cell contains atoms centered on the sides of the unit cell. Combining 7 Crystal Classes (cubic, tetragonal, orthorhombic, hexagonal, monoclinic, triclinic, trigonal) with 4 unit cell types (P, I, F, C) symmetry allows for only 14 types of 3-D lattice. 3
- 4. (c) 2003 Brooks/Cole Publishing / Thomson Learning™ Lattice parameters in cubic, orthorhombic and hexagonal crystal systems. 4
- 5. Define basic terms and give examples of each: • Points (atomic positions) • Vectors (defines a particular direction - plane normal) • Miller Indices (defines a particular plane) • relation to diffraction • 3-index for cubic and 4-index notation for HCP Basic definitions – Lattice sites 5
- 6. Miller indices - A shorthand notation to describe certain crystallographic directions and planes in a material. Denoted by [ ], <>, ( ) brackets. A negative number is represented by a bar over the number. Points, Directions and Planes in the Unit Cell 6
- 7. • Coordinates of selected points in the unit cell. • The number refers to the distance from the origin in terms of lattice parameters. Point Coordinates 7
- 8. 8 Point Coordinates Point coordinates for unit cell center are a/2, b/2, c/2 ½½½ Point coordinates for unit cell corner are 111 Translation: integer multiple of lattice constants identical position in another unit cell z x y a b c 000 111 y z 2c b b
- 9. 9 Crystallographic Directions 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a, b, and c 3. Adjust to smallest integer values 4. Enclose in square brackets, no commas [uvw] ex: 1, 0, ½ => 2, 0, 1 => [201 ] -1, 1, 1 z x where overbar represents a negative index [ 111]=> y [ 111] [ 201] Algorithm
- 10. Procedure: 1. Any line (or vector direction) is specified by 2 points. • The first point is, typically, at the origin (000). 2. Determine length of vector projection in each of 3 axes in units (or fractions) of a, b, and c. • X (a), Y(b), Z(c) 1 1 0 3. Multiply or divide by a common factor to reduce the lengths to the smallest integer values, u v w. 4. Enclose in square brackets: [u v w]: [110] direction. a b c DIRECTIONS will help define PLANES (Miller Indices or plane normal). [ 1 10]5. Designate negative numbers by a bar • Pronounced “bar 1”, “bar 1”, “zero” direction. 6. “Family” of [110] directions is designated as <110>. Directions in a Crystal 10
- 11. Examples 210 X = ½ , Y = ½ , Z = 1 [½ ½ 1] [1 1 2] X = 1 , Y = ½ , Z = 0 [1 ½ 0] [2 1 0] 11
- 12. 12 • When we write the direction [n1n2n3] depending on the origin, negative directions are written as R = n1a1 + n2a2 + n3a3 To specify the direction, the smallest possible integers must be used. Y direction (origin) O - Y direction X direction - X direction Z direction - Z direction ][ 321 nnn Negative Directions 12
- 13. 13 X = -1 , Y = -1 , Z = 0 [110] X = 1 , Y = 0 , Z = 0 [1 0 0] Examples of Crystal Directions 13
- 14. 14 X =-1 , Y = 1 , Z = -1/6 [-1 1 -1/6] [6 6 1] A vector can be moved to the origin. Examples 14
- 15. 15 • Within a crystal lattice it is possible to identify sets of equally spaced parallel planes. These are called lattice planes. • In the figure, the density of lattice points on each plane of a set is the same & all lattice points are contained on each set of planes. b a b a The set of planes for a 2D lattice. Crystal Planes 15
- 16. • If the plane passes through origin, either: – Construct another plane, or – Create a new origin – Then, for each axis, decide whether plane intersects or parallels the axis. • Algorithm for Miller indices 1. Read off intercepts of plane with axes in terms of a, b, c 2. Take reciprocals of intercepts 3. Reduce to smallest integer values 4. Enclose in parentheses, no commas. 16 Crystallographic Planes
- 17. 17 Crystallographic Planes • Crystallographic planes are specified by 3 Miller Indices (h k l). All parallel planes have same Miller indices.
- 18. 18 • Miller Indices are a symbolic vector representation for the orientation of an atomic plane in a crystal lattice & are defined as the reciprocals of the fractional intercepts which the plane makes with the crystallographic axes. • To find the Miller indices of a plane, take the following steps: 1. Determine the intercepts of the plane along each of the three crystallographic directions. 2. Take the reciprocals of the intercepts. 3. If fractions result, multiply each by the denominator of the smallest fraction. 18
- 19. 19 Crystallographic Planesz x y a b c 4. Miller Indices (110) example a b c z x y a b c 4. Miller Indices (200) 1. Intercepts 1 1 2. Reciprocals 1/1 1/1 1/ 1 1 0 3. Reduction 1 1 0 1. Intercepts 1/2 2. Reciprocals 1/½ 1/ 1/ 2 0 0 3. Reduction 2 0 0 example a b c
- 20. 20 Crystallographic Planes z x y a b c 4. Miller Indices (634) example 1. Intercepts 1/2 1 3/4 a b c 2. Reciprocals 1/½ 1/1 1/¾ 2 1 4/3 3. Reduction 6 3 4
- 21. x y z Intercepts Intercept in terms of lattice parameters Reciprocals Reductions Enclosure a -b c/2 -1 1/2 0 -1 2 N/A (012) Determine the Miller indices for the plane shown in the sketch 21
- 22. 22 Family of Planes • Planes that are crystallographically equivalent have the same atomic packing. • Also, in cubic systems only, planes having the same indices, regardless of order and sign, are equivalent. • Ex: {111} = (111), (111), (111), (111), (111), (111), (111), (111) (001)(010), (100), (010),(001),Ex: {100} = (100), _ __ __ _ __ _ _ _ _
- 24. FCC Unit Cell with (110) plane 24
- 25. BCC Unit Cell with (110) plane 25
- 26. 26 Axis X Y Z Intercept points 1 ∞ ∞ Reciprocals 1/1 1/ ∞ 1/ ∞ Smallest Ratio 1 0 0 Miller İndices (100) Example-1 (1,0,0)
- 27. 27 Axis X Y Z Intercept points 1 1 ∞ Reciprocals 1/1 1/ 1 1/ ∞ Smallest Ratio 1 1 0 Miller İndices (110) Example-2 (1,0,0) (0,1,0)
- 28. 28 Axis X Y Z Intercept points 1 1 1 Reciprocals 1/1 1/ 1 1/ 1 Smallest Ratio 1 1 1 Miller İndices (111)(1,0,0) (0,1,0) (0,0,1) Example-3
- 29. 29 Axis X Y Z Intercept points 1/2 1 ∞ Reciprocals 1/(½) 1/ 1 1/ ∞ Smallest Ratio 2 1 0 Miller İndices (210) (1/2, 0, 0) (0,1,0) Example-4
- 30. 30 Miller Indices Reciprocal numbers are: 2 1 , 2 1 , 3 1 Plane intercepts axes at cba 2,2,3 Indices of the plane (Miller): (2,3,3) (200) (100) Indices of the direction: [2,3,3]a 3 2 2 b c [2,3,3] Z X Y (100) Z X Y (110) Z X Y (111)
- 31. 31 Example 7 31
- 32. SUMMARY • Crystallographic points, directions and planes are specified in terms of indexing schemes. • Materials can be single crystals or polycrystalline. • Material properties generally vary with single crystal orientation (anisotropic), but are generally non- directional (isotropic) in polycrystals with randomly oriented grains. • Some materials can have more than one crystal structure. This is referred to as polymorphism (or allotropy). 32
- 33. A crystal resides in real space. The diffraction pattern of the crystal in Fraunhofer diffraction geometry resides in Reciprocal Space. In a diffraction experiment (powder diffraction using X-rays, selected area diffraction in a TEM), a part of this reciprocal space is usually sampled. From the real lattice the reciprocal lattice can be geometrically constructed. The properties of the reciprocal lattice are ‘inverse’ of the real lattice → planes ‘far away’ in the real crystal are closer to the origin in the reciprocal lattice. As a real crystal can be thought of as decoration of a lattice with motif; a reciprocal crystal can be visualized as a Reciprocal Lattice decorated with a motif* of Intensities. Reciprocal Crystal = Reciprocal Lattice + Intensities as Motif* The reciprocal of the ‘reciprocal lattice’ is nothing but the real lattice! Planes in real lattice become points in reciprocal lattice and vice-versa. Reciprocal Lattice
- 34. In diffraction patterns (Fraunhofer geometry) (e.g. SAD), planes are mapped as spots (ideally points). Hence, we would like to have a construction which maps planes in a real crystal as points. Apart from the use in ‘diffraction studies’ we will see that it makes sense to use reciprocal lattice when we are dealing with planes. Motivation for constructing reciprocal lattices As the index of the plane increases → the interplanar spacing decreases → and ‘planes start to crowd’ around the origin in the real lattice (refer figure). Hence, we work in reciprocal lattice when dealing with planes. As seen in the figure the diagonal is divided into (h + k) parts.
- 35. Let us start with a one dimensional lattice and construct the reciprocal lattice Reciprocal Lattice Real Lattice How is this reciprocal lattice constructed Note in 1D planes are points and have Miller indices of single digit (they have been extended into the second dimension (as lines) for better visibility The plane (2) has intercept at ½, plane (3) has intercept at 1/3 etc. As the index of the plane increases it gets closer to the origin (there is a crowding towards the origin) One unit cell Reciprocal Lattice Each one of these points correspond to a set of ‘planes’ in real space Note that in reciprocal space index has NO brackets Real Lattice
- 36. Reciprocal Lattice (01) (10) (11) (21) 10 20 11 221202 01 21 00 The reciprocal lattice has an origin! 1a 2a 1a 1 1 a * 11g * 21g* b2 * b1 Now let us construct some 2D reciprocal lattices Example-1 Each one of these points correspond to a set of ‘planes’ in real space 2 1 a Note that vectors in reciprocal space are perpendicular to planes in real space (as constructed!) But do not measure distances from the figure! Overlay of real and reciprocal lattices g vectors connect origin to reciprocal lattice points
