IB Chemistry on Lewis structure, ionic and covalent bonding
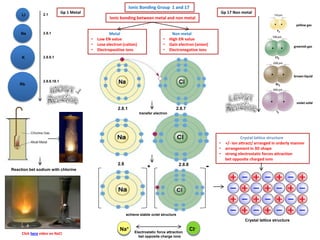
- 1. brown liquid violet solid Ionic Bonding Group 1 and 17 Gp 1 Metal2.1 2.8.1 2.8.8.1 2.8.8.18.1 Na Li K Rb Click here video on NaCI yellow gas greenish gas Ionic bonding between metal and non metal Metal • Low EN value • Lose electron (cation) • Electropositive ions Non metal • High EN value • Gain electron (anion) • Electronegative ions 2.8.1 2.8.7 Gp 17 Non metal transfer electron 2.8 2.8.8 achieve stable octet structure + - Na+ CI- Electrostatic force attraction bet opposite charge ions Crystal lattice structure • +/- ion attract/ arranged in orderly manner • arrangement in 3D shape • strong electrostatic forces attraction bet opposite charged ions Reaction bet sodium with chlorine Crystal lattice structure
- 2. violet solid brown liquid Gp 2 Metal 2.2 2.8.2 2.8.8.2 2.8.8.18.2 Mg Be Ca Sr Click here simulation ionic bonding yellow gas greenish gas Ionic bonding between metal and non metal Metal • Low EN value • Lose electron (cation) • Electropositive ions Non metal • High EN value • Gain electron (anion) • Electronegative ions 2.8.2 2.7 Gp 17 Non metal transfer electron 2.8 achieve stable octet structure Mg2+ F- Electrostatic force attraction bet opposite charge ions Crystal lattice structure Crystal lattice structure • +/- ion attract/arrange orderly manner • arrangement in 3D shape • strong electrostatic forces attraction bet opposite charged ions 2.8. 82.8 F-
- 3. Cations/Metals/+ve ions Gp1 Gp 2 Gp3 Transition metals ions ( variable oxidation states) Oxidation state +1 Oxidation state +2 Oxidation state +3 Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Zn +2 Li 1+ Be2+ Sc 3+ Ti 2+ Ti3+ V 2+ V 3+ Cr 2+ C r3+ Cr6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co2+ Ni2+ Cu1+ Cu2+ Zn2+ Na 1+ Mg2+ Al 3+ K 1+ Ca2+ Anion/Non metal Gp 5 Gp 6 Gp 7 Oxidation state Oxidation state Oxidation state -3 -2 -1 N3- O2- F-1 P3- S2- CI-1 Br-1 I-1 Metal/Cations/+ve ion Non Metal/ Anions/ -ve ion Ionic Compound Li2O MgCI2 Al2O3 FeO NiO CuO Li3N Mg3N2 AlN Fe3N2 Ni3N2 Cu3N2 Oxidation state/Charge ion → Li1+ O2- Formula compound Li2 O1 Video on polyatomic ions Writing Chemical Formula Ionic Compounds Step 1 : Write Oxidation state/charge Step 2 : Balance it, (electrically neutral) by cross multiply – as subscript
- 4. Polyatomic ions Group of non-metals bonded together Oxidation state Oxidation state Oxidation state -1/+1 -2 -3 (OH)-1 Hydroxide (SO4)2- Sulphate (PO4)3- Phosphate (CN)-1 Cyanide (SO3)2- Sulphite (SCN)-1 Thiocyanate (CO3)2- Carbonate (NO3)-1 Nitrate (S2O3)2- Thiosulphate (NO2)-1 Nitrite (Cr2O7)2- Dichromate (NH4)+1 Ammonium Polyatomic ions Li2(CO3) Mg(CO3) Al2(CO3)3 Fe(CO3) Ni(CO3) Cu(CO3) Li(OH) Mg(OH)2 Al(OH)3 Fe(OH)2 Ni(OH)2 Cu(OH)2 Li2(SO4) Mg(SO4) Al2(SO4)3 FeSO4 Ni(SO4) Cu(SO4) Video on polyatomic ions Ionic Compound Metal/Cations/+ve ions Cations/Metals/+ve ions Gp1 Gp 2 Gp3 Transition metals ions ( variable oxidation states) Oxidation state +1 Oxidation state +2 Oxidation state +3 Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Li 1+ Be2+ Sc 3+ Ti 2+ Ti3+ V 2+ V 3+ Cr 2+ C r3+ Cr6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co2+ Ni2+ Cu1+ Cu2+ Na 1+ Mg2+ Al 3+ K 1+ Ca2+ Oxidation state/Charge ion → Li1+ (CO3)2- Formula compound Li2 (CO3 )1 Step 1 : Write Oxidation state/charge ion Step 2 : Balance it, (electrically neutral) by cross multiply – as subscript Writing Chemical Formula Ionic Compounds
- 5. Acids Alkali Metal Hydroxide Metal oxides Salts Gas HCI Hydrochloric acid KOH Potassium hydroxide CuO Copper(II) oxide CaCO3 Calcium carbonate CO Carbon monoxide HNO3 Nitric acid NaOH Sodium hydroxide MgO Magnesium oxide Na2CO3 Sodium carbonate CO2 Carbon dioxide H2SO3 Sulphurous acid Ca(OH)2 Calcium Hydroxide ZnO Zinc oxide NaHCO3 Sodium bicarbonate SO2 Sulphur dioxide HCOOH Methanoic acid NH3 Ammonia Na2O Sodium oxide KNO3 Potassium nitrate SO3 Sulphur trioxide CH3COOH Ethanoic acid Mg(OH)2 Magnesium hydroxide Al2O3 Aluminium oxide Pb(NO3)2 Lead (II) Nitrate NO2 Nitrogen dioxide H3PO4 Phosphoric acid Cu(OH)2 Copper (II) hydroxide Fe2O3 Iron(III) oxide NaNO3 Sodium nitrate CH4 Methane H2CO3 Carbonic acid Al(OH)3 Aluminium hydroxide K2S Potassium sulphide PbI2 Lead (II) nitrate H2S Hydrogen sulphide HNO2 Nitrous acid Fe(OH)2 Iron (II) hydroxide PbS Lead(II) sulphide AgCI Silver chloride O2 Oxygen HF Hydrofluoric acid Fe(OH)3 Iron (III) hydroxide ZnS Zinc sulphide MgSO4 Magnesium sulphate N2 Nitrogen HCIO Hypochlorous acid Zn(OH)2 Zinc hydroxide AI2S3 Aluminium sulphide Na2S2O3 Sodium thiosulphate CI2 Chlorine Chemical Formula for common chemicals Naming chemical compoundWriting chemical formulaWriting chemical formula VIDEO TUTORIALS
- 6. violet solid brown liquid Click here simulation on covalent bond yellow gas greenish gas Covalent bonding between non metals 2.8.7 Gp 17 Non metal achieve stable octet structure CI shared pair electron Covalent Bonding Electrostatic forces attraction between nucleus with shared pair electron 2.8.8 2.8.7 Sharing electron Gp 17 Non metal 2.8.8 CI Non metal • High EN value • Gain electron (anion) • Electronegative ions Covalent Bond Group 17 CICI Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: :. x x X x x x x CI CI :: x x x x CI CI Non metal • High EN value • Gain electron (anion) • Electronegative ions Single covalent bond – shared pair electron
- 7. Covalent bonding between non metals Covalent Bonding Single covalent bond ONE pair shared e 2.8.8 2.8.8 Covalent Bond Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: : : . x x X X x x x x x x O CI CI:: x x x x x x CI CI Sharing electron Bond Strength 2.8 2.8 Sharing electron O O: x x O N O O N: Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron C : :. N N: N N : Covalent Bonding Double covalent bond TWO pair shared e Covalent Bonding Triple covalent bond THREE pair shared e O x x: CO CO O O O Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Covalent Bonding Double covalent bond TWO pair shared e
- 8. : CI .. x Bond Bond order Bond strength Bond length/pm C - C 1 347 154 C = C 2 612 134 C Ξ C 3 820 120 N - N 1 159 145 N = N 2 418 123 N Ξ N 3 914 110 Bond length and Bond strength Bond length = 0.199nm Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron CI CI: x x :: : : . x x X X x x x x O CI CI:: x x x x x x CI CI O O: x x O N O O N: Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron Lewis structure/diagram . Electron cross dot . Valence/bonding pair electron O : : . N N: N N Triple bond > Double bond > Single bond Bonding pair e -involve in bonding Bond length decrease Bond strength Increase (Double/Triple bond) Bond length = 0.121nm Bond length = 0.110nm Bond order up – Bond strength up – Bond length down O Non bonding pair (Lone pair electron) Bonding pair electron C O: : Bonding pair electron Dative bond (electron pair of oxygen) Types of bonding Lone pair e –not involve in bonding Dative/coordinate bond - pair e come from an atom Exception to octet rule All period 2 element - observe octet rule except Be and B Electron deficient Less than 8 valence e Expanded octet More than 8 valence e All period 3 element - observe octet rule except P and S : BeCI CIx . : :: :: . X x. Be - 4 valence e BCI CI : :: : :: x ::B - 6 valence e P S CI CI CI CI CI CI CICI CICI CI P - 10 valence e S – 12 valence e
- 9. O ONO C N C BeH N B X x C SSO Linear O C H H NO N O Trigonal planar C OO CH N HH Be C N N O O:x x X x X x X X X X X X X x X x + + : : . F F F Electron deficient Be Dative bond from N NCx x : Electron deficient N (7 valence electron) H H O O O O O O C O O O O N O O B XF F F C H H x x O S O O S O O::: : : O C O x x O N 2- - 2- O : : : x x O : : : O - Electron deficient B O S O : : O: : :: Dative bond from S : ON O O : : - O X X || || Sulphur expanded octet (12 valence electron) ✓ BF3 CH2 O SO3 CO3 2- NO3 - CO2 HCN BeH2 CN - NO2 + NO :: X X X X Dative bond from N
- 10. O O O O O O : : N : : O O O N O S O O OO S : S O O OO S : : N O O N OO - - O O N O O N : - Dative bond from N electron deficient N (7 valence electron) S O O : : Sulphur expanded octet (10 valence electron) or or Dative bond from S NO2 -NO2 SO2O3 O HH H2O H H O O F2O F F F F O S CICI SCI2 SH2 S H H S CI CI S H NH2 - N HH HH N - - H: : : : : : : : : : : All molecule – octet rule Bend Bend O O : O Dative bond from O
- 11. || : S CI Sulphur expanded octet (12 valence electron) C H H CH4 NH4 + N BH4 - PCI4 + POCI3 P CI -+ Tetrahedral H H H H H H B H H HH CI CICI + P O CI CICI C H H H H H N H H H + H B H H H - CI P CI CI CI + : : : : : :: : : : : : O :: P CI CI :: : : : : : : O SO4 2- O O O O S O :: O || O H2SO4 H3PO4 CIO4 - || O S O S O O :: O || P OH O :: P || O CI O O O O CI :: OO O - - Phosphorus expanded octet (10 valence electron) Chlorine expanded octet (14 valence electron) Tetrahedral 2- 2-
- 12. N H N : : O SO3 2-PH3NH3 O HH H2O H H O O F2O F F F F O S CICI SCI2 SH2 S H H S CI CI S H NH2 - N HH HH N - - H: : : : : : : : : : : Two lone pair central atom Bend CIO3 - H H P H H H H H H P H H H : O O S O S O : O 2- 2- CI O O O CI : O O O - : - SOCI2 S CI CI O S : O CI CI : : : :: : One lone pair central atom One lone pair Pyrimidal Two lone pair CH4 C H NH4 + BH4 - PCI4 + POCI3 H H H H C H H H N H H H H H N H H H H B HH H H B H H H CI CI CI CI P CI CI CI P CI O- - + + + + P CI CICI || CI CI CI O P :: Tetrahedral NO lone pair : : : : :: :: : :: : :: :: : : :: : NO lone pair central atom
- 13. P CI CI CI CI CI : P CI CI CI CI Seesaw structure T structure S F F F F S F F F F Te CI CI CI CI Te CI CI CI CI : CI F F F CI F F F I CI CI CI CI I CI CI I F F F F F I F F F Xe F F O O Xe F F O O : + + : CI Linear structure I I I Xe CI CI I F F ::::::: : : :: :: :: : : : : : :: : : :: : : : : : : : :: : :::: : : :: : :: : :: :: : :: : :: : :: :: :: :: :: :: :: :: :: : : : : NO lone pair NO lone pair central atom ONE lone pair : ONE lone pair central atom TWO lone pair TWO lone pair central atom THREE lone pair THREE lone pair central atom : BrF F F Br F F F : : : : : : :: : - PCI5 SF4 TeCI4 (IF4)+ XeO2F2 CIF3 ICI3 BrF3 (I3)- (ICI2)- XeF2 Trigonal bipyrimidal Central atom S, Te, I, Xe - expanded octet - 10 valence electron Central atom P - expanded octet - 10 valence electron Central atom CI, I, Br - expanded octet - 10 valence electron Central atom I and Xe - expanded octet - 10 valence electron -
- 14. Octahedral F S SF6 F F F F F PCI6 - P CI CI CI CI CI CI IF5O I O || F F F F F F S F F F F F CI P CI CI CI CI CI I F F F F F :: O NO lone pair Square pyrimidal CI Sb Sb CI CI CICI CI CI CICI CI (SbCI5)2- BrF5 Xe F FF F Xe F XeF4 F F F F Br F FF F F Br F F O F F Xe || F FF F O :: Xe F F F F F Te F FF F F Te F F F F XeOF4 (TeF5)- - - CICI I CICI (ICI4)- - I CI CI CI CI -Square planar : : : : ::: ONE lone pair TWO lone pair : : :: : : : :: ::: : :: : :::: :: : : : : : : : :: : : : : :: : ::: : : : : :: : :: : :::: : :::: :::: : : : :: :: : :: : : :::: :::: : : ::: :::: : :: : : : : : : :: ::: :::: : 2- 2- :::: :::: : : : : :: :: :::: :::: :: :: NO lone pair central atom Central atom S, P , I - expanded octet - 12 valence electron ONE lone pair central atom Central atom Sb, Br, Xe, Te - expanded octet - 12 valence electron TWO lone pair central atom Central atom Xe, I - expanded octet - 12 valence electron - -
- 15. Chemical Bonds Achieve stable electron arrangement of noble gases Ionic Bonds Transfer of electron from metal to non metal Metal donate e Non Metal accept e Positive ion (cation) Negative ion (anion) Ionic compound Solubility Covalent Bonds Sharing of electron bet non metal atoms Covalent compound/molecules Non Metal share electrons Difference in physical property m/p + b/pconductivityVolatility + -electrostatic forces attraction Concept Map Volatility Solubility Conductivity M/p B/p Ionic compound Low Soluble in aqueous solvent High (In aqueous) High Covalent compound High Soluble in organic solvent Low Low 0 0.4 4 Difference in electronegativity difference < 0.4 covalent compound difference > 2 ionic compound 2 CI-Na+ EN - 0.9 EN - 3.0 Diff = 3 – 0.9 = 2.1 C 2.5 H 2.1 EN – 2.5 EN – 2.1 Diff = 2.5 – 2.1 = 0.4
