Atomic Bonding, Ionic, Covalent, Metallic Bonds, Physical Science Lesson PowerPoint
- 1. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +)
- 3. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 4. -Nice neat notes that are legible and use indents when appropriate. -Example of indent. -Skip a line between topics - -Make visuals clear and well drawn. Label please. Neutron Proton Electron
- 5. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 11. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up.
- 12. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up.
- 13. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 14. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 15. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 16. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 17. This is really difficult learning ahead and I’m going to try my best to learn it. I’m not going to give up. This is really difficult and I’m going to quit as soon as I don’t know it. I’m going to check out completely or create issues for those choosing A.
- 18. New Area of focus: Atomic Bonding Copyright © 2010 Ryan P. Murphy
- 19. New Area of focus: Atomic Bonding Copyright © 2010 Ryan P. Murphy
- 20. Chemical Bonding: The attraction that holds atoms close to each other. Copyright © 2010 Ryan P. Murphy
- 21. Chemical Bonding: The attraction that holds atoms close to each other. Copyright © 2010 Ryan P. Murphy
- 22. Ionic, Covalent, Metallic Copyright © 2010 Ryan P. Murphy
- 23. Ionic, Covalent, Metallic Copyright © 2010 Ryan P. Murphy
- 24. Ionic, Covalent, Metallic Covalent – Share electrons Copyright © 2010 Ryan P. Murphy
- 25. Ionic, Covalent, Metallic Covalent – Share electrons Copyright © 2010 Ryan P. Murphy
- 26. Ionic, Covalent, Metallic Covalent – Share electrons Ionic – Gain or lose electrons (transfer) Copyright © 2010 Ryan P. Murphy
- 27. Ionic, Covalent, Metallic Covalent – Share electrons Ionic – Gain or lose electrons (transfer) Copyright © 2010 Ryan P. Murphy
- 28. Ionic, Covalent, Metallic Covalent – Share electrons Ionic – Gain or lose electrons (transfer) Metallic- Many free electrons Copyright © 2010 Ryan P. Murphy
- 29. “My name is Bond.” Copyright © 2010 Ryan P. Murphy
- 30. “Covalent Bond.” Copyright © 2010 Ryan P. Murphy
- 31. Covalent bonding occurs by a sharing of valence electrons Copyright © 2010 Ryan P. Murphy
- 32. Covalent bonding occurs by a sharing of valence electrons Copyright © 2010 Ryan P. Murphy
- 33. Covalent bonding occurs by a sharing of valence electrons (Strongest) Copyright © 2010 Ryan P. Murphy
- 34. Covalent bonding occurs by a sharing of valence electrons (Strongest) (SPONCH). Copyright © 2010 Ryan P. Murphy
- 38. Ionic bonding (+/-) Bonds created by the attraction of opposite charges. Copyright © 2010 Ryan P. Murphy
- 41. Ionization: The process of removing electrons from an atom to form ions. Copyright © 2010 Ryan P. Murphy
- 42. • Ionic - One atom strips electron from the other so both are now stable. Held then by + / - charge Copyright © 2010 Ryan P. Murphy
- 43. • Ionic - One atom strips electron from the other so both are now stable. Held then by + / - charge Copyright © 2010 Ryan P. Murphy
- 44. • Ionic - One atom strips electron from the other so both are now stable. Held then by + / - charge Copyright © 2010 Ryan P. Murphy
- 50. Ionic Bonding: Forms crystal lattice. Copyright © 2010 Ryan P. Murphy
- 52. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 53. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 54. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy Metal or non-metal?
- 55. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 56. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy Metal or non-metal?
- 57. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 58. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 59. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 60. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 61. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy
- 62. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy Opposites sides of the Periodic Table. Gives an electron +1
- 63. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy Opposites sides of the Periodic Table. Gives an electron +1 Gains an electron -1
- 64. Metal bonding to a non-metal will always be an ionic bond. Copyright © 2010 Ryan P. Murphy Opposites sides of the Periodic Table. Gives an electron +1 Gains an electron -1
- 65. Video: Ionic and Covalent Bonds http://www.youtube.com/watch?v=Kj3o0Xv hVqQ&feature=results_main&playnext=1& list=PL85B1E4851BDEE325
- 66. Metallic bonding: The bonding between atoms within metals. The sharing of many free electrons. Copyright © 2010 Ryan P. Murphy
- 67. Metallic bonding: The bonding between atoms within metals. The sharing of many free electrons. Copyright © 2010 Ryan P. Murphy Learn more: http://www.chemguide.co.uk/atoms/bondin g/metallic.html
- 68. • Activity! Generating heat by breaking metallic bonds. Copyright © 2010 Ryan P. Murphy
- 69. • Activity! Generating heat by breaking metallic bonds. Wear Safety Goggles. Copyright © 2010 Ryan P. Murphy
- 70. • Activity! Generating heat by breaking metallic bonds. – Bend spoon back and forth to generate very hot temperatures, WATCH OUT! Copyright © 2010 Ryan P. Murphy
- 71. • Activity! Generating heat by breaking metallic bonds. – Bend spoon back and forth to generate very hot temperatures, WATCH OUT! – Do not try this in the lunchroom! Copyright © 2010 Ryan P. Murphy
- 72. • Video! Ionic and Covalent Bonding. • http://www.youtube.com/watch?v=QqjcCv zWwww
- 73. • Video Link! (Optional) Khan Academy, Atomic Bonding. – http://www.khanacademy.org/video/ionic-- covalent--and-metallic-bonds?playlist=Chemistry
- 74. Ion: A charged atom. When an atom strips an electron, now one atom has 1+ (cation), and the other has 1 – (anion), Copyright © 2010 Ryan P. Murphy
- 75. Ion: A charged atom. When an atom strips an electron, now one atom has 1+ (cation), and the other has 1 – (anion), Copyright © 2010 Ryan P. Murphy
- 76. Ion: A charged atom. When an atom strips an electron, now one atom has 1+ (cation), and the other has 1 – (anion), Copyright © 2010 Ryan P. Murphy “+1 Cation, Animal hoarding adds Cats. I love cats, Cats are positive.
- 77. Ion: A charged atom. When an atom strips an electron, now one atom has 1+ (cation), and the other has 1 – (anion), Copyright © 2010 Ryan P. Murphy
- 78. Ion: A charged atom. When an atom strips an electron, now one atom has 1+ (cation), and the other has 1 – (anion), Copyright © 2010 Ryan P. Murphy
- 79. The closer and more tightly bound an electron is to the nucleus, the more difficult it will be to remove, and the higher its ionization energy will be.
- 80. Protons stink! I hate being in this shell. This is the worst. Nightmare
- 81. Protons stink! I hate being in this shell. This is the worst. Nightmare
- 82. Protons stink! I hate being in this shell. This is the worst. Nightmare This is so nice I’m so happy.
- 83. Protons stink! I hate being in this shell. This is the worst. Nightmare This is so nice I’m so happy.
- 85. The atom has a neutral charge when the number is the same.
- 86. The atom has a neutral charge when the number is the same. When you remove an electron
- 87. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive
- 88. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive Yay, we lost Grumpy. I feel so more positive.
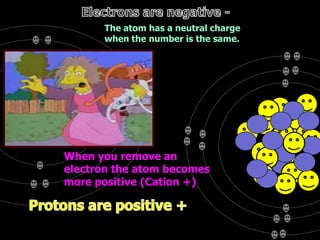
- 89. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +) Yay, we lost Grumpy. I feel so more positive.
- 90. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +)
- 91. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +)
- 92. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +) When you add an electron the atom becomes more negative.
- 93. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +) When you add an electron the atom becomes more negative. Anion -
- 94. The atom has a neutral charge when the number is the same. When you remove an electron the atom becomes more positive (Cation +) When you add an electron the atom becomes more negative. Anion - More negativity
- 95. • Which atom below is the anion, and which is the cation?
- 96. • Sodium formed a cation because it lost 1 electron and became positive.
- 97. • Sodium formed a cation because it lost 1 electron and became positive. Add cats, Cats are +
- 98. • Chlorine formed an anion because it gained -1 electron. More negative. Add cats, Cats are +
- 99. • Which atom below formed a cation, and which formed an anion?
- 100. • Which atom below formed a cation, and which formed an anion?
- 101. • Which atom below formed a cation, and which formed an anion?
- 102. • Which atom below formed a cation, and which formed an anion?
- 103. • Which atom below formed a cation, and which formed an anion?
- 104. • Which Gnome is the Cation, and which Gnome is the Anion? Copyright © 2010 Ryan P. Murphy
- 105. • Which Gnome is the Cation, and which Gnome is the Anion? Copyright © 2010 Ryan P. Murphy Cation +1 gives an electron
- 106. • Which Gnome is the Cation, and which Gnome is the Anion? Copyright © 2010 Ryan P. Murphy Cation +1 gives an electron
- 107. • Which Gnome is the Cation, and which Gnome is the Anion? Copyright © 2010 Ryan P. Murphy Cation +1 gives an electron Anion -1 accepts an electron
- 108. • Electron Affinity: The amount of energy required to detach an electron from a singly charged negative ion. Copyright © 2010 Ryan P. Murphy
- 109. • Will this atom want to lose this valence electron, or gain many electrons to have a full outer shell? Copyright © 2010 Ryan P. Murphy
- 110. • Answer: This Potassium atom will want to lose this electron. It has a low electron affinity. Copyright © 2010 Ryan P. Murphy
- 111. Copyright © 2010 Ryan P. Murphy Who wants it?
- 112. Copyright © 2010 Ryan P. Murphy Who wants it?
- 113. Copyright © 2010 Ryan P. Murphy Who wants it?
- 114. Copyright © 2010 Ryan P. Murphy Who wants it?
- 115. Copyright © 2010 Ryan P. Murphy Who wants it? It is ionic because it's a bond between a metal(potassium) and a non-metal(chlorine). Potassium has one electron in its valence shell, and chlorine has seven electrons in its valence shell. Following the octet rule, the potassium gives an electron to the chlorine. Then the negatively charged chlorine ion and the positively charged potassium ion stick together because of their opposite charges. Ionic bonds give electrons, covalent bonds share electrons Do we want to know the difficult explanation beneath this box or skip it? Why?
- 116. Copyright © 2010 Ryan P. Murphy Who wants it? It is ionic because it's a bond between a metal(potassium) and a non-metal(chlorine). Potassium has one electron in its valence shell, and chlorine has seven electrons in its valence shell. Following the octet rule, the potassium gives an electron to the chlorine. Then the negatively charged chlorine ion and the positively charged potassium ion stick together because of their opposite charges. Ionic bonds give electrons, covalent bonds share electrons Do we want to know the difficult explanation beneath this box or skip it? Why?
- 117. Copyright © 2010 Ryan P. Murphy Who wants it? It is ionic because it's a bond between a metal(potassium) and a non-metal(chlorine). Potassium has one electron in its valence shell, and chlorine has seven electrons in its valence shell. Following the octet rule, the potassium gives an electron to the chlorine. Then the negatively charged chlorine ion and the positively charged potassium ion stick together because of their opposite charges. Ionic bonds give electrons, covalent bonds share electrons
- 118. • Will this atom want to lose these valence electrons, or gain one electron to have a full outer shell? Copyright © 2010 Ryan P. Murphy
- 119. • Answer: This Chlorine atom will want to gain one electron rather than lose seven. Copyright © 2010 Ryan P. Murphy
- 120. • Answer: This Chlorine atom will want to gain one electron rather than lose seven. – It has a high electron affinity. Copyright © 2010 Ryan P. Murphy
- 121. • Answer: This Chlorine atom will want to gain one electron rather than lose seven. – It has a high electron affinity. Copyright © 2010 Ryan P. Murphy Learn more: Ionization. http://www.wisegeek.com/what- is-ionization.htm
- 122. Which atom below has a high electron affinity, and which has a low electron affinity? Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 123. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 124. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 125. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 126. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 127. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 128. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 129. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 130. Answers: Fluorine Sodium High Electron Affinity Low Electron Affinity Copyright © 2010 Ryan P. Murphy
- 131. • Precipitation Reactions: Occur when cations and anions of aqueous solutions combine to form an insoluble ionic solid, called a precipitate. Copyright © 2010 Ryan P. Murphy
- 132. • Precipitation Reactions: Occur when cations and anions of aqueous solutions combine to form an insoluble ionic solid, called a precipitate. Copyright © 2010 Ryan P. Murphy See Video (2 minutes) http://www.youtube.com/watch?v=8RmVwz2fNGc First - AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) Second- AgNO3(aq) + NaI(aq) → AgI(s) + NaNO3(aq)
- 133. • Video Link! Precipitation Reactions Crash Course. – Advanced and Optional – http://www.youtube.com/watch?v=IIu16dy3ThI&li st=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQQ8oGr
- 134. • Acids and Bases optional PowerPoint in folder. – Nice reading can be found here. – http://www.visionlearning.com/library/module_ viewer.php?mid=58
- 135. • Acid: a substance which when added to water produces hydrogen ions [H+]. – React with zinc, magnesium, or aluminum and form hydrogen (H2(g)) – React with compounds containing CO3 2- and form carbon dioxide and water – Turn litmus red – Taste sour (lemons contain citric acid, for example) – Tasting Acids in the lab would be unsafe.
- 136. • Acid: a substance which when added to water produces hydrogen ions [H+]. – React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). – React with compounds containing CO3 2- and form carbon dioxide and water. – Turn litmus red. – Taste sour (lemons contain citric acid, for example). – Tasting Acids in the lab would be unsafe.
- 137. • Base: a substance which when added to water produces hydroxide ions [OH-]. – Feel soapy or slippery – Turn litmus blue – They react with most cations to precipitate hydroxides – Taste bitter like soap. – Do not taste in the lab.
- 138. • Base: a substance which when added to water produces hydroxide ions [OH-]. – Feel soapy or slippery. – Turn litmus blue. – They react with most cations to precipitate hydroxides. – Taste bitter like soap. – Do not taste in the lab.
- 139. • Which is an Acid and which is a Base? OH- OH- OH- OH- OH- Lots of OH-, High pH
- 140. • Which is an Acid and which is a Base? OH- OH- OH- OH- OH- Lots of OH-, High pH
- 141. • Which is an Acid and which is a Base? OH- OH- OH- OH- OH- Lots of OH-, High pH
- 142. • Which is an Acid and which is a Base? OH- OH- OH- OH- OH- Lots of OH-, High pH
- 143. • Which is an Acid and which is a Base?
- 144. • Which is not true of a base? A.) Feel soapy or slippery. B.) Turns litmus red. C.) They react with most cations to precipitate hydroxides. D.) Taste bitter like soap. – Do not taste in the lab.
- 145. • Which is not true of a base? A.) Feel soapy or slippery. B.) Turns litmus red. C.) They react with most cations to precipitate hydroxides. D.) Taste bitter like soap. – Do not taste in the lab.
- 146. • Which is not true of a base? A.) Feel soapy or slippery. B.) Turns litmus red. C.) They react with most cations to precipitate hydroxides. D.) Taste bitter like soap. – Do not taste in the lab.
- 147. • Which is not true of a base? A.) Feel soapy or slippery. B.) Turns litmus red. C.) They react with most cations to precipitate hydroxides. D.) Taste bitter like soap. – Do not taste in the lab.
- 148. • Which is not true of a base? A.) Feel soapy or slippery. B.) Turns litmus blue. C.) They react with most cations to precipitate hydroxides. D.) Taste bitter like soap. – Do not taste in the lab.
- 150. • Which is not true of acids? A.) Acid: a substance which when added to water produces hydrogen ions [H+]. B.) React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). C.) They react with most cations to precipitate hydroxides D.) Turn litmus red. E.) Taste sour (lemons contain citric acid, for example). • Tasting Acids in the lab would be unsafe.
- 151. • Which is not true of acids? A.) Acid: a substance which when added to water produces hydrogen ions [H+]. B.) React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). C.) They react with most cations to precipitate hydroxides D.) Turn litmus red. E.) Taste sour (lemons contain citric acid, for example). • Tasting Acids in the lab would be unsafe.
- 152. • Which is not true of acids? A.) Acid: a substance which when added to water produces hydrogen ions [H+]. B.) React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). C.) They react with most cations to precipitate hydroxides D.) Turn litmus red. E.) Taste sour (lemons contain citric acid, for example). • Tasting Acids in the lab would be unsafe.
- 153. • Which is not true of acids? A.) Acid: a substance which when added to water produces hydrogen ions [H+]. B.) React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). C.) They react with most cations to precipitate hydroxides D.) Turn litmus red. E.) Taste sour (lemons contain citric acid, for example). • Tasting Acids in the lab would be unsafe.
- 154. • Which is not true of acids? A.) Acid: a substance which when added to water produces hydrogen ions [H+]. B.) React with zinc, magnesium, or aluminum and form hydrogen (H2(g)). C.) React with compounds containing CO3 2- and form carbon dioxide and water D.) Turn litmus red. E.) Taste sour (lemons contain citric acid, for example). • Tasting Acids in the lab would be unsafe.
- 156. • Electronegativity increases from lower left to upper right. Copyright © 2010 Ryan P. Murphy
- 157. • Electronegativity increases from lower left to upper right. Copyright © 2010 Ryan P. Murphy Moving top to bottom down the periodic table, electronegativity decreases.
- 158. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Copyright © 2010 Ryan P. Murphy
- 159. Copyright © 2010 Ryan P. Murphy Note: Noble gases are missing.
- 160. Copyright © 2010 Ryan P. Murphy
- 161. • The most strongly electronegative element, Fluorine (F). Copyright © 2010 Ryan P. Murphy
- 162. • The most strongly electronegative element, Fluorine (F). Copyright © 2010 Ryan P. Murphy “I want electrons .”
- 163. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy
- 164. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away electrons.”
- 165. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away electrons.” “I want to gain electrons”
- 166. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away electrons.” “I want to gain electrons” “You guys should get together.”
- 167. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. Copyright © 2010 Ryan P. Murphy
- 168. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy
- 169. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Those elements attract electrons like wicked.”
- 170. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Not the Noble Gases however.”
- 171. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Not the Noble Gases however.” “They’re wicked different.”
- 172. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 173. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 174. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 175. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 176. • A polar bond: Results in the unequal sharing of the electrons in the bond. Copyright © 2010 Ryan P. Murphy
- 177. • A polar bond: Results in the unequal sharing of the electrons in the bond. – When two unlike atoms are covalently bonded, the shared electrons will be more strongly attracted to the atom of greater electronegativity Copyright © 2010 Ryan P. Murphy
- 178. • A polar bond: Results in the unequal sharing of the electrons in the bond. – When two unlike atoms are covalently bonded, the shared electrons will be more strongly attracted to the atom of greater electronegativity Copyright © 2010 Ryan P. Murphy The presence or absence of polar bonds within a molecule plays a very important part in determining chemical and physical properties of those molecules. Some of these properties are melting points, boiling points, viscosity and solubility in solvents.
- 185. • Hydrogen Bond: A chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom.
- 186. • Hydrogen Bond: A chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom.
- 187. • Hydrogen Bond: A chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom. – Especially a nitrogen, oxygen, or flourine atom of another molecule.
- 188. • Hydrogen Bond: A chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom. – Especially a nitrogen, oxygen, or flourine atom of another molecule.
- 198. • The three classes of bonds
- 199. • The three classes of bonds – Nonpolar Covalent
- 200. • The three classes of bonds – Nonpolar Covalent – Polar Covalent
- 201. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic
- 202. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element.
- 203. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar.
- 204. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference
- 205. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference Hydrogen = 2.20 Oxygen = 3.44
- 206. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 =
- 207. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24
- 208. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24
- 209. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. H2O Electron Negativity Difference Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24
- 210. Try Ethane C2H6?
- 211. Try Ethane C2H6?
- 212. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. C2H6 Ethane Electron Negativity Diff.
- 213. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. C2H6 Ethane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55
- 214. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. C2H6 Ethane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 =
- 215. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. C2H6 Ethane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35
- 216. • The three classes of bonds – Nonpolar Covalent – Polar Covalent – Ionic • The most commonly used electronegativity scale is Pauling's. Most Periodic Tables gives the value for each element. – Differences 1.7 or greater, the bond is usually ionic, – Differences Less than 1.7, the bond is usually covalent, » Unless the difference is less than 0.5 the bond has some degree of polarity – Differences of less than 0.5 are considered to be nonpolar. C2H6 Ethane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35
- 222. • Which one is polar covalent and which one nonpolar?
- 223. • Which one is polar covalent and which one nonpolar?
- 224. • Which one is polar covalent and which one nonpolar?
- 225. • Which one is polar covalent and which one nonpolar?
- 226. • Which one is polar covalent and which one nonpolar?
- 227. • Which one is polar covalent and which one nonpolar?
- 228. • Which one is polar covalent and which one nonpolar?
- 229. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 230. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 231. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 232. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 233. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 234. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 235. • Layering liquids with different densities. • Use a clear container and add the following in this order…. – Corn Syrup – Water (food Coloring) – Vegetable Oil
- 236. • I would recommend completing these questions right away.
- 238. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44
- 239. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Do we want to see the answers?
- 240. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Do we want to see the answers?
- 241. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers?
- 242. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 243. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 244. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 245. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 246. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 247. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 248. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 249. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Do we want to see the answers? Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 250. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 251. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 252. Carbon = 2.55 Hydrogen = 2.20 Carbon = 2.55 Oxygen = 3.44 Hydrogen = 2.20 Oxygen = 3.44 Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Oxygen = 3.44 Carbon = 2.55 3.44 – 2.55 = .89 Hydrogen = 2.20 Oxygen = 3.44 3.44 – 2.20 = 1.24 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 253. • Video! Ionic and Covalent Bonding. • http://www.youtube.com/watch?v=QqjcCv zWwww
- 254. • Video Link! Ionic and Covalent Bonds – https://www.youtube.com/watch?v=7DjsD7Hcd9U
- 255. • (Optional Link): Khan Academy • Ionization Energy (12 min) Advanced • http://www.khanacademy.org/video/periodi c-table-trends--ionization- energy?playlist=Chemistry
- 256. • Video Link! Speaking Chemistry Crash Course. – Optional and Advanced. – http://www.youtube.com/watch?v=mlRhLicNo8Q&l ist=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQQ8oGr
- 257. • Remember: • Covalent – Sharing an Electron many of the SPONCH elements.
- 258. • Remember: • Covalent – Sharing an Electron many of the SPONCH elements. • Ionic – Opposite charges + / -
- 259. • Remember: • Covalent – Sharing an Electron many of the SPONCH elements. • Ionic – Opposite charges + / - • Metallic – Many electrons
- 267. • Quiz Wiz: Label as either… • Covalent, Ionic, or Metallic 1-10 Copyright © 2010 Ryan P. Murphy
- 279. • Bonus: Name the actor and movie character.
- 280. • Answers! Quiz Wiz: Label as either, Covalent, Ionic, or Metallic 1-10 Copyright © 2010 Ryan P. Murphy
- 301. • Bonus: Name the actor and movie character.
- 302. • Bonus: Name the actor and movie character. Sean Connery
- 303. • Optional worksheets / quizzes associated with atomic bonding (Links) – http://moodle.northsideprep.org/pluginfile.php/ 8956/mod_resource/content/0/Electronegativit y-ws1-nh4.pdf – Atomic Bonding Electronegativity Differences – Atomic Bonding and Electronegativity Sheet
- 304. • Optional PowerPoint “Properties of Water” – Found in activities folder. – More about polar and nonpolar.
- 305. • Video Link! Carbon Crash Course and Nice Review of Unit. (Optional) – Preview for language and content. – http://www.youtube.com/watch?v=QnQe0xW_J Y4&list=EC3EED4C1D684D3ADF
- 306. Chemical Change: The change of substances into other substances through a reorganization of the atoms. Copyright © 2010 Ryan P. Murphy
- 307. • Check out this awesome chemical reaction
- 309. • Demonstrations of chemical change.
- 310. • Demonstration – Chemical Change with baking soda and vinegar. Copyright © 2010 Ryan P. Murphy
- 311. • Demonstration – Fill bottle with screw top with vinegar. – Fill balloon with baking soda using funnel. – Attach bottom of balloon to top of bottle making sure that no baking soda enters bottle. – Turn balloon upright so contents fall into bottle and observe. Copyright © 2010 Ryan P. Murphy
- 312. • The baking soda (NaHCO3) mixes with the vinegar (HC2H3O2) –HC2H3O2 + NaHCO3 ===> NaC2H3O2 + H2CO3 –The H2CO3 quickly breaks down • H2CO3 ===> H2O + CO2 • Carbon dioxide can be used to put out fire. Copyright © 2010 Ryan P. Murphy
- 313. • The baking soda (NaHCO3) mixes with the vinegar (HC2H3O2) –HC2H3O2 + NaHCO3 ===> NaC2H3O2 + H2CO3 –The H2CO3 quickly breaks down • H2CO3 ===> H2O + CO2 • Carbon dioxide can be used to put out fire. Copyright © 2010 Ryan P. Murphy
- 314. • The baking soda (NaHCO3) mixes with the vinegar (HC2H3O2) –HC2H3O2 + NaHCO3 ===> NaC2H3O2 + H2CO3 –The H2CO3 quickly breaks down • H2CO3 ===> H2O + CO2 • Carbon dioxide can be used to put out fire. Copyright © 2010 Ryan P. Murphy
- 315. • The baking soda (NaHCO3) mixes with the vinegar (HC2H3O2) –HC2H3O2 + NaHCO3 ===> NaC2H3O2 + H2CO3 –The H2CO3 quickly breaks down • H2CO3 ===> H2O + CO2 • Carbon dioxide can be used to put out fire. Copyright © 2010 Ryan P. Murphy
- 316. • The baking soda (NaHCO3) mixes with the vinegar (HC2H3O2) –HC2H3O2 + NaHCO3 ===> NaC2H3O2 + H2CO3 –The H2CO3 quickly breaks down • H2CO3 ===> H2O + CO2 • Carbon dioxide can be used to put out fire. Copyright © 2010 Ryan P. Murphy
- 317. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy
- 318. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy Please set-up the following. Dissolve about a spoonful of salt into warm water.
- 319. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy Please set-up the following. Dissolve about a spoonful of salt into warm water. Connect one end on the positive side of the battery and the other to the tip of the pencil.
- 320. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy Please set-up the following. Dissolve about a spoonful of salt into warm water. Connect one end on the positive side of the battery and the other to the tip of the pencil. Do the same for the negative side connecting it to the second pencil top. Place the other two ends of the pencil into the salt water.
- 321. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy Please set-up the following. Dissolve about a spoonful of salt into warm water. Connect one end on the positive side of the battery and the other to the tip of the pencil. Do the same for the negative side connecting it to the second pencil top. Place the other two ends of the pencil into the salt water. Observe Bubbles that form at the bottom of the pencil in the water.
- 322. • Activity Demonstration – Electrolysis. – What is happening in the beaker? Can you guess the chemical change? Copyright © 2010 Ryan P. Murphy Please set-up the following. Dissolve about a spoonful of salt into warm water. Connect one end on the positive side of the battery and the other to the tip of the pencil. Do the same for the negative side connecting it to the second pencil top. Place the other two ends of the pencil into the salt water. Observe Bubbles that form at the bottom of the pencil in the water. This is hydrogen gas.
- 323. • Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen (H).
- 324. • Water and hydrogen could be the answer to finding a clean renewable source of energy.
- 325. • Combustion can create a chemical change. Copyright © 2010 Ryan P. Murphy
- 326. • Combustion can create a chemical change. –Requires oxygen which then mixes with the substance being burned. Copyright © 2010 Ryan P. Murphy
- 328. • Chemical Reaction Mercury(II) thiocyanate decomposition. – http://www.youtube.com/watch?v=ritaljhhk7s Copyright © 2010 Ryan P. Murphy
- 331. http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Areas of Focus within The Atoms and Periodic Table Unit: Atoms (Atomic Force Microscopes), Rutherford’s Gold Foil Experiment, Cathode Tube, Atoms, Fundamental Particles, The Nucleus, Isotopes, AMU, Size of Atoms and Particles, Quarks, Recipe of the Universe, Atomic Theory, Atomic Symbols, #’;s, Valence Electrons, Octet Rule, SPONCH Atoms, Molecules, Hydrocarbons (Structure), Alcohols (Structure), Proteins (Structure), Atomic Bonds, Ionic Bonds, Covalent Bonds, Metallic Bonds, , Precipitation Reactions, Acids and Bases, Electron Negativity, Polar Bonds, Chemical Change, Exothermic Reactions, Endothermic Reactions, Laws Conservation of Matter, Balancing Chemical Equations, Oxidation and Reduction, Periodic Table of the Elements, Organization of Periodic Table, Transition Metals, Acids and Bases, Non-Metals, Metals, Metalloids, Ionization.
- 337. • This PowerPoint roadmap is one small part of my Atoms and Periodic Table Unit. • This unit includes a four part 2000+ slide PowerPoint roadmap. • 13 page bundled homework that chronologically follows slideshow • 14 pages of unit notes with visuals. • 3 PowerPoint review games. • Activity sheets, rubrics, advice page, curriculum guide, materials list, and much more. • http://sciencepowerpoint.com
- 339. • Please visit the links below to learn more about each of the units in this curriculum – These units take me about four years to complete with my students in grades 5-10. Earth Science Units Extended Tour Link and Curriculum Guide Geology Topics Unit http://sciencepowerpoint.com/Geology_Unit.html Astronomy Topics Unit http://sciencepowerpoint.com/Astronomy_Unit.html Weather and Climate Unit http://sciencepowerpoint.com/Weather_Climate_Unit.html Soil Science, Weathering, More http://sciencepowerpoint.com/Soil_and_Glaciers_Unit.html Water Unit http://sciencepowerpoint.com/Water_Molecule_Unit.html Rivers Unit http://sciencepowerpoint.com/River_and_Water_Quality_Unit.html = Easier = More Difficult = Most Difficult 5th – 7th grade 6th – 8th grade 8th – 10th grade
- 340. Physical Science Units Extended Tour Link and Curriculum Guide Science Skills Unit http://sciencepowerpoint.com/Science_Introduction_Lab_Safety_Metric_Methods. html Motion and Machines Unit http://sciencepowerpoint.com/Newtons_Laws_Motion_Machines_Unit.html Matter, Energy, Envs. Unit http://sciencepowerpoint.com/Energy_Topics_Unit.html Atoms and Periodic Table Unit http://sciencepowerpoint.com/Atoms_Periodic_Table_of_Elements_Unit.html Life Science Units Extended Tour Link and Curriculum Guide Human Body / Health Topics http://sciencepowerpoint.com/Human_Body_Systems_and_Health_Topics_Unit.html DNA and Genetics Unit http://sciencepowerpoint.com/DNA_Genetics_Unit.html Cell Biology Unit http://sciencepowerpoint.com/Cellular_Biology_Unit.html Infectious Diseases Unit http://sciencepowerpoint.com/Infectious_Diseases_Unit.html Taxonomy and Classification Unit http://sciencepowerpoint.com/Taxonomy_Classification_Unit.html Evolution / Natural Selection Unit http://sciencepowerpoint.com/Evolution_Natural_Selection_Unit.html Botany Topics Unit http://sciencepowerpoint.com/Plant_Botany_Unit.html Ecology Feeding Levels Unit http://sciencepowerpoint.com/Ecology_Feeding_Levels_Unit.htm Ecology Interactions Unit http://sciencepowerpoint.com/Ecology_Interactions_Unit.html Ecology Abiotic Factors Unit http://sciencepowerpoint.com/Ecology_Abiotic_Factors_Unit.html
- 342. • The entire four year curriculum can be found at... http://sciencepowerpoint.com/ Please feel free to contact me with any questions you may have. Thank you for your interest in this curriculum. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com
