24a synthesis
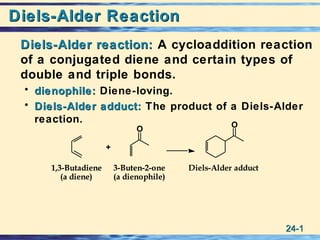
- 1. 24-24-11 Diels-Alder ReactionDiels-Alder Reaction Diels-Alder reaction:Diels-Alder reaction: A cycloaddition reaction of a conjugated diene and certain types of double and triple bonds. • dienophile:dienophile: Diene-loving. • Diels-Alder adduct:Diels-Alder adduct: The product of a Diels-Alder reaction. Diels-Alder adduct3-Buten-2-one (a dienophile) 1,3-Butadiene (a diene) + O O 3-Buten-2-one (a dienophile) 1,3-Butadiene (a diene) + O O
- 2. 24-24-22 Diels-Alder ReactionDiels-Alder Reaction • Alkynes also function as dienophiles. • Cycloaddition reaction:Cycloaddition reaction: A reaction in which two reactants add together in a single step to form a cyclic product. Diels-Alder adductDiethyl 2-butynedioate (a dienophile) + 1,3-butadiene (a diene) COOEt COOEt COOEt COOEt
- 3. 24-24-33 Diels-Alder ReactionDiels-Alder Reaction • We write a Diels-Alder reaction in the following way: • The special value of D-A reactions are that they: 1. form six-membered rings. 2. form two new C-C bonds at the same time. 3. are stereospecific and regioselective. Note the reaction of butadiene and ethylene gives only traces of cyclohexene. Diene Dieno- phile Adduct
- 4. 24-24-44 Diels-Alder ReactionDiels-Alder Reaction • The conformation of the diene must be s-cis. s-trans conformation (lower in energy) s-cis conformation (higher in energy)
- 5. 24-24-55 Diels-Alder Reaction Steric RestrictionsDiels-Alder Reaction Steric Restrictions • (2Z,4Z)-2,4-Hexadiene is unreactive in Diels- Alder reactions because nonbonded interactions prevent it from assuming the planar s-cis conformation. (2Z,4Z)-2,4-Hexadiene s-trans conformation (lower energy) s-cis conformation (higher energy) methyl groups forced closer than allowed by van der Waals radii
- 6. 24-24-66 Diels-Alder ReactionDiels-Alder Reaction • Reaction is facilitated by a combination of electron-withdrawing substituents on one reactant and electron-releasing substituents on the other. CyclohexeneEthylene1,3-Butadiene 200°C pressure 3-Buten-2-one 140°C + 1,3-Butadiene O O + 2,3-Dimethyl- 1,3-butadiene + 30°C 3-Buten-2-one O O
- 7. 24-24-77 Diels-Alder ReactionDiels-Alder Reaction Electron-Withdrawing Groups Electron-Releasing Groups -C N (cyano) - OR (ether) - OOCR (ester) - CHO (aldehyde, ketone) - COOH (carboxyl) - COOR (ester) - NO2 (nitro) - CH3, alkyl groups
- 8. 24-24-88 Diels-Alder ReactionDiels-Alder Reaction • The Diels-Alder reaction can be used to form bicyclic systems. + room temperature 170°C Diene Dienophile Dicyclopentadiene (endo form) H H
- 9. 24-24-99 Diels-Alder ReactionDiels-Alder Reaction • Exo and endo are relative to the double bond derived from the diene. the double bond derived from the diene endo (inside) exo (outside) relative to the double bond
- 10. 24-24-1010 Diels-Alder ReactionDiels-Alder Reaction • For a Diels-Alder reaction under kinetic control, endo orientation of the dienophile is favored. Methyl bicyclo[2.2.1]hept-5-en- endo-2-carboxylate (racemic) Methyl propenoate Cyclopentadiene + OCH3 O H COOCH3 COOCH3 redraw 1 2 3 45 6 7
- 11. 24-24-1111 Diels-Alder ReactionDiels-Alder Reaction • The configuration of the dienophile is retained. COOCH3 COOCH3 COOCH3 COOCH3 A cis dienophile) Dimethyl cis-4-cyclohexene- 1,2-dicarboxylate + COOCH3 H3 COOC COOCH3 COOCH3 A trans dienophile) Dimethyl trans-4-cyclohexene- 1,2-dicarboxylate (racemic) +
- 12. 24-24-1212 Diels-Alder ReactionDiels-Alder Reaction • The configuration of the diene is retained. CH3 CH3 CH3 O O O O O O O O O H3 C H3 C O O OH3C H3 C CH3 + + H H H H Check that this is endo.
- 13. 24-24-1313 Diels-Alder ReactionDiels-Alder Reaction Mechanism • No evidence for the participation of either radical of ionic intermediates. • Chemists propose that the Diels-Alder reaction is a concerted pericyclic reaction. Pericyclic reactionPericyclic reaction: A reaction that takes place in a single step, without intermediates, and involves a cyclic redistribution of bonding electrons. Concerted reaction: All bond making and bond breaking occurs simultaneously.
- 14. 24-24-1414 Diels-Alder ReactionDiels-Alder Reaction • Mechanism of the Diels-Alder reaction
- 15. 24-24-1515 Aromatic Transition StatesAromatic Transition States Hückel criteria for aromaticity:Hückel criteria for aromaticity: The presence of (4n + 2) pi electrons in a ring that is planar and fully conjugated. Just as aromaticity imparts a special stability to certain types of molecules and ions, the presence of (4n + 2) electrons in a cyclic transition state imparts a special stability to certain types of transition states. • Reactions involving 2, 6, 10, 14.... electrons in a cyclic transition state have especially low activation energies and take place particularly readily.
- 16. 24-24-1616 Aromatic Transition States,Aromatic Transition States, ExamplesExamples • Decarboxylation of β-keto acids and β- dicarboxylic acids. • Cope elimination of amine N-oxides. O O H O O H C O O O CO2+ enol of a ketone (A cyclic six-membered transition state) O heat + A cyclic six-membered transition state N,N-dimethyl- hydroxylamine C C H N CH3 CH3 N CH3 CH3 O HC C An alkene +
- 17. 24-24-1717 Aromatic Transition StatesAromatic Transition States • the Diels-Alder reaction • pyrolysis of esters (Problem 22.42) We now look at examples of two more reactions that proceed by aromatic transition states: • Claisen rearrangement. • Cope rearrangement. Diene Dieno- phile Adduct
- 18. 24-24-1818 Claisen RearrangementClaisen Rearrangement Claisen rearrangement:Claisen rearrangement: A thermal rearrangement of allyl phenyl ethers to 2- allylphenols. Allyl phenyl ether 200-250°C 2-Allylphenol O OH
- 19. 24-24-1919 Claisen RearrangementClaisen Rearrangement O Allyl phenyl ether heat OH o-Allylphenol O H A cyclohexadienone intermediate keto-enol tautomerism O Transition state
- 20. 24-24-2020 Cope RearrangementCope Rearrangement Cope rearrangement:Cope rearrangement: A thermal isomerization of 1,5-dienes. 3,3-Dimethyl- 1,5-hexadiene 2-Methyl-2,6- heptadiene heat
- 21. 24-24-2121 Cope RearrangementCope Rearrangement Example 24.8Example 24.8 Predict the product of these Cope rearrangements. (a) (b) 350°C OH H 320°C
- 22. 24-24-2222 Synthesis of Single EnantiomersSynthesis of Single Enantiomers • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzymes, which create a chiral environment in which reaction takes place. • Enzymes show high enantiomeric and diastereomeric selectivity with the result that enzyme-catalyzed reactions invariably give only one of all possible stereoisomers.
- 23. 24-24-2323 Synthesis of Single EnantiomersSynthesis of Single Enantiomers How do chemists achieve the synthesis of single enantiomers? The most common method is to produce a racemic mixture and then resolve it. How? • the different physical properties of diastereomeric salts. • the use of enzymes as resolving agents. • chromatographic on a chiral substrate.
- 24. 24-24-2424 Synthesis of Single EnantiomersSynthesis of Single Enantiomers • In a second strategy, asymmetric inductionasymmetric induction, the achiral starting material is placed in a chiral environment by reacting it with a chiral auxiliarychiral auxiliary. Later it will be removed. • E. J. Corey used this chiral auxiliary to direct an asymmetric Diels-Alder reaction. • 8-Phenylmenthol was prepared from naturally occurring enantiomerically pure menthol. Me HO Me Me Me HO Me Me Ph 8-Phenylmenthol (an enantiomerically pure chiral auxillary) Menthol (enantiomerically pure) several steps
- 25. 24-24-2525 Synthesis of Single EnantiomersSynthesis of Single Enantiomers • The initial step in Corey’s prostaglandin synthesis was a Diels-Alder reaction. • By binding the achiral acrylate with enantiomerically pure 8-phenylmenthol, he thus placed the dienophile in a chiral environment. • The result is an enantioselective synthesis. OBn Me O Me Me Ph O ORO BnO RO O OBn + Diels-Alder + Enantiomerically pure 97% 3% 89% Achiral
- 26. 24-24-2626 Synthesis of Single EnantiomersSynthesis of Single Enantiomers • A third strategy is to begin a synthesis with an enantiomerically pure starting material. • Gilbert Stork began his prostaglandin synthesis with the naturally occurring, enantiomerically pure D-erythrose. • This four-carbon building block has the R configuration at each stereocenter. • With these two stereocenters thus established, he then used well understood reactions to synthesize his target molecule in enantiomerically pure form. HO H O OH OH D-Erythrose
