003 properties of_pure_substance
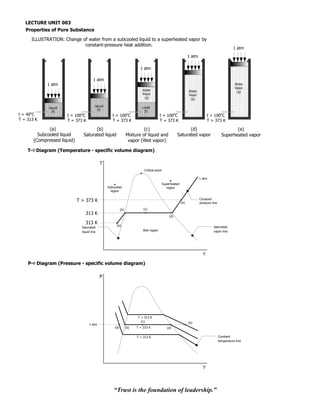
- 1. LECTURE UNIT 003 Properties of Pure Substance ILLUSTRATION: Change of water from a subcooled liquid to a superheated vapor by constant-pressure heat addition. 1 atm 1 atm 1 atm 1 atm 1 atm Water Vapor Water Water (g) Vapor Vapor (g) (g) Liquid Liquid Liquid (f) (f) o (f) t = 40 C o t = 100 C t = 100 C o t = 100oC t > 100oC T = 313 K T = 373 K T = 373 K T = 373 K T > 373 K (a) (b) (c) (d) (e) Subcooled liquid Saturated liquid Mixture of liquid and Saturated vapor Superheated vapor (Compressed liquid) vapor (Wet vapor) T-v Diagram (Temperature - specific volume diagram) T Critical point 1 atm Superheated Subcooled region region Constant T > 373 K (e) pressure line (b) (c) 313 K (d) 313 K (a) Saturated Saturated liquid line Wet region vapor line v P-v Diagram (Pressure - specific volume diagram) P T > 313 K (c) (e) 1 atm (a) (b) T = 373 K (d) T = 313 K Constant temperature line v “Trust is the foundation of leadership.”
- 2. Quality, x If a substance exist as part liquid and part vapor at the saturation temperature, its quality is defined as the ratio of mass of vapor to the total mass. Note that the definition of quality assumes a homogeneous mixture of vapor and liquid, mass of vapor x= (mass of vapor + mass of liquid) To find the specific enthalphy, specific entropy, specific volume and specific internal energy of point c. h = hf + x hfg hfg = (hg - hf) s = sf + x sfg sfg = (sg - sf) v = vf + x vfg vfg = (vg - vf) u = uf + x ufg ufg = (ug - uf) Critical Point At this point, the saturated liquid and saturated vapor are identical. For Water Critical temperature: t = 374.14oC Critical Pressure: P = 22.09 Mpa Critical volume: v = 0.003155 m3/kg PROBLEM SET: 1. Complete the following table by using Steam Tables and illustrate the points in a T-v diagram and P-v diagram. t oC P kPa x% h (kJ/kg) u (kJ/kg) v (m3/kg) State a 200 852.45 b c 300 150 80 1000 d 300 800 e 300 0.85 f 210 5000 Indicate for each state whether it is subcooled liquid, saturated liquid, mixture, saturated vapor, or superheated vapor. 2. A 2-m3 tank contains a saturated vapor at 40oC. Determine the pressure and mass in the tank if the substance is steam. [ ] 3. Steam has a quality of 90% at 200oC. Determine (a) the enthalpy (b) the specific volume. [ ] 4. A 500-liter tank contains a saturated mixture of steam and water at 300oC. Determine (a) the mass of each phase if their volumes are equal (b) the volume occupied by each phase if their masses are equal. [ ] 5. Determine the volume occupied by 3 kg of water at 1000 kPa and temperature 1000oC. [ ] 6. A rigid tank contains 3 kg of saturated steam at a pressure of 3000 kPa. Because of heat transfer to the surroundings, the pressure decreases to 1000 kPa. Determine (a) the T-v diagram and P-v diagram (b) the tank’s volume and the quality of steam at the final state. [ ] 7. A 20-liter tank contains saturated mixture of water and vapor at 100 kPa. The volume occupied by the liquid is 20 cm3. Heat is added until all the water evaporates and the tank contains only saturated vapor. Determine (a) the T-v diagram and P-v diagram (b) the pressure at this final state. [ ] 8. What must be the quality of the steam at 2000 kPa such that it will reach the critical state if heated at constant volume. [ ] 9. A 1-ft3 tank contains a saturated mixture of water and vapor at 1 atm. The volume occupied by the liquids is 20 in3. Heat is added until all water evaporates and the tank contains only saturated vapor. Determine the pressure at this final state. [ ] 10. Determine the volume occupied by 5 lbm of water at 100 psia and temperatures of 100oF and 500oF. [ ] “Fear of ideas makes us impotent and ineffective.”