Essay question(structure atom)
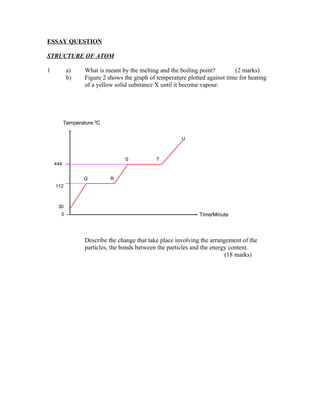
- 1. ESSAY QUESTION STRUCTURE OF ATOM 1 a) What is meant by the melting and the boiling point? (2 marks) b) Figure 2 shows the graph of temperature plotted against time for heating of a yellow solid substance X until it become vapour. Temperature 0C U S T 444 Q R 112 30 0 Time/Minute Describe the change that take place involving the arrangement of the particles, the bonds between the particles and the energy content. (18 marks)
- 2. 2 (a) Figure 3 shows the arrangement of electrons for element M J Figure 3 i. Name 3 subatomic particle and give the relative mass and relative charge for each particle mentioned. (3marks) ii. What is meant by electron valence? What is the electron valence for the atom of element M? (2marks) iii. Give the proton number and the nucleon number of M. (1marks) iv. What is meant by electron configuration?Give the electron configuration for the atom of element M. (2marks) Atom Proton number Nucleon number X 6 12 Y 6 14 (b) i. With the help of the information given in the table 3, compare atom X with atom Y. (4marks) ii. Which of the above atoms is radioactive?Give two uses of the atoms stated. (3marks)
- 3. ANSWER
- 4. 1 (a) - The melting point is a fixed temperature when the solid of a substance changes to a liquid at definite pressure (1 marks) - The boiling point is a fixed temperature when the liquid state of a substance change to the gaseous state at a definite presure (1 marks) (b) - substance X is in the solid state at a temperature up to Q. (1 marks) - The particles are arranged orderly and are compact. (1 marks) - The force between the particles are strong (1 marks) - the particles jus vibrate. (1 marks) -When it is heated until time Q, the energy absorbed is strong enough to overcome the attractive force between the particles. (1 marks) -The bond break and the particles begin to move. (1 marks) - Solid X begins to melt and changes to become a liquid. (1 marks) - Between the time of Q and R, substance X is in solid and liquid state. (1 marks) - Although heating continues, the temperature does not rise.(1 marks) - The energy absorbed is used to overcome the attractive force and causes the substance to change from solid to liquid. (1 marks) - The fixed temperature is known as the melting point. (1 marks) - After R minutes the process of melting is complete and at this time all the solid becomes liquid. (1 marks) - When heating continues for a time to exceed R minutes, the temperature increases. (1 marks) - For the time exceeding R, the energy absorbed is enough to overcome the attractive force between the liquid particles in substance X. (1 marks) - The bond break and the particles begin to move freely becoming gas or vapour of the sunstances X. (1 marks) - For the time between S and T , Substances X is in the liquid and gas state. (1 marks) - When heating continues the temperature does not change.(1 marks) - The energy absorbed is used to overcome the attractive forces between the particles and the substances changes from liquid to gas state. (1 marks) - The fixed temperature at this stage is known as the boiling point. (1 marks) - After T, the process of boiling is complete and at this time, all the substance X has become gas. (1 marks)
- 5. 2 (a) i. Subatomic Relative mass Relative charge particle Proton 1 +1 Neutron 1 Neutral Electron 1/1840 -1 ii. - Electron valence is the number of the number of the electrons found in the outermost shell of an atom. (1 marks) - The electron valence of the atom of element M is 1. (1 marks) iii. Proton number : 11 Nucleon number : 23 iv. – The electron configuration is the distribution of electrons showing how the electrons of an atom of an element are located in the shells surrounding the nucleus. - The electron configuration of element M is 2.8.1 (b) i - Both atoms have the same number of proton of 6
- 6. - Atom X has 6 neutron while atom Y has 8 neutrons - Both atoms are of the same element because the number of proton of both atom is the same. - Both atoms are ispotopes because they have the same number of protons but have different number of neutrons ii. Atom Y Uses of atom Y : - Detecting the pathway of carbon during photosynthesis - To determine the date of archeological artifacts
