Chemical Equilibrium
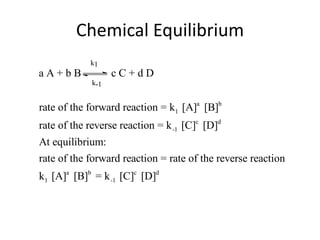
- 3. Chemical Equilibrium When equilibrium, a dynamic state, is reached the concentrations of A, B, C, and D are invariant (i.e., no longer change) The reaction continues in a dynamic state, but the concentrations remain the same. The rate of the forward reaction is equal to the rate of the reverse reaction.
- 4. Chemical EquilibriumConcentration versus time at equilibrium [C] and [D] [A] and [B]
- 5. Chemical EquilibriumRate versus time at equilibrium
- 6. Consider the Reaction between Hydrogen and Iodine If the initial concentrations of hydrogen and iodine gases are 0.0175 M, calculate the equilibrium concentrations of the reactants and products if the equilibrium constant is 55.64 at 425oC.
- 7. a = 51.64; b = -1.95; and c = 0.0170
- 8. If x = 1.37 x 10-2 M x = 2.41 x 10-2 M is not possible, because it would lead to negative matter!
- 9. The equilibrium expression does not include solids For example, the equilibrium expression for the following reaction:
- 10. The equilibrium expression when water is involved For example,
- 11. Kc versus Kp
- 12. From PV = nRT
- 14. Consider the Following Reaction:
- 15. Consider the Following Reaction:
- 16. Manipulating the Equilibrium Expression:
- 17. Manipulating the Equilibrium Expression:
- 18. Manipulating the Equilibrium Expression: If Keq equals 3.5 x 108 for Calculate Keq for
- 19. Manipulating the Equilibrium Expression:
- 20. Finding K for a Reaction from Other K values
- 21. Finding K for a Reaction from Other K values
- 22. Finding K for a Reaction from Other K values
- 23. Calculate Kp at 226.8oC for Reaction (4) given Reactions (1), (2), and (3):
- 24. Kp at 226.8oC for
- 25. Kp at 226.8oC for
- 26. Kp at 226.8oC for
- 27. The Reaction Quotient, Q Q is obtained by using a similar formula as K, but Q may not be at equilibrium. The reaction is at dynamic equilibrium when Q = Keq If Q is greater than Keq, then the reaction is not at equilibrium and must adjust so that product(s) will go to reactant(s) until a state of dynamic equilibrium is reached If Q is less than Keq, then the reaction is not at equilibrium and must adjust so that more reactant(s) will go to product(s) until a state of dynamic equilibrium is reached
- 28. Application of Q For the following equilibrium: Keq= 2.5 at 25oC is the reaction at equilibrium when [CH3CH2CH2CH3] = 0.97 M and [(CH3)2CHCH3] = 2.18M? If not, calculate the equilibrium concentrations.
- 29. Application of Q
- 30. Application of Q Since Q (2.25) is less than Keq (2.5) Therefore, the equilibrium concentration of CH3CH2CH2CH3 is 0.90 M (0.97 – 0.07) and the equilibrium concentration of (CH3)2CHCH3 is 2.25M (2.18 + 0.07)
- 31. Another Application of Q For the following equilibrium: Keq= 1.7 x 10-3 is the reaction at equilibrium when [N2] = 0.50 M , [O2] = 0.25 M, and [NO] = 0.0042 M? If not, calculate the equilibrium concentrations.
- 32. Another Application of Q For the following equilibrium: Q (0.000141) is less than Keq (0.0017)
- 33. Another Application of Q 0.0042+2x 0.50 M - x 0.25 - x 2 + N N O O ( g ) ( g ) ( g ) 2 2 x = 5.1 x 10-3
- 34. Another Application of Q x = 5.1 x 10-3
- 35. Let’s calculate the equilibrium constant for the following reaction at 1000 K: 1.00 mol of SO2 and 1.00 mol of O2 are placed in a 1.00 L flask. When equilibrium is achieved 0.925 mol of SO2 is formed.
- 36. Let’s calculate the KP for the following reaction at 800 K: A tank contains hydrogen sulfide gas at 10.00 atm at 800 K. When equilibrium is achieved, the partial pressure of S2 is 2.0 x 10-2 atm. What is Kcfor this reaction?
- 37. Let’s calculate the Kc for the following reaction: If 0.050 mol of trans-1,2-diodocyclohexane is dissolved in carbon tetrachloride to make a 1.00 L solution. At equilibrium the concentration of I2 is 0.035 M. What is Kpfor this reaction?
- 38. Kc for the following reaction is 1.0 x 10-5 at 1227oC: Calculate the equilibrium concentrations if 0.80 mole of N2 and 0.20 mole of O2 are placed initially in a 2.00 L flask at 1227oC. Calculate Kp at 1227oC, and calculate the equilibrium pressures at N2, O2, and NO at 1227oC.
- 39. Le Chatelier’s Principle When a system in dynamic equilibrium encounters stress, the system will adjust itself in order to relieve the stress.
- 40. The Stresses Change the temperature Change the concentration of reactants or products Change the volume Change the pressure (only when there is a difference between the number of moles of gaseous reactants and products)
- 41. Changing the Temperature Adjustment in the equilibrium is determined by whether the reaction is endothermic or exothermic. If the reaction is exothermic, an increase in temperature shifts the equilibrium toward the reactants. A decrease in temperature shifts the reaction toward the products.
- 42. Changing the Temperature If the reaction is endothermic, an increase in temperature shifts the equilibrium toward the products. A decrease in temperature shifts the reaction toward the reactants. Changes in temperature result in changes in the value of the equilibrium constant.
- 43. For example, the following data were obtained for changes in the equilibrium Constant with changes in temperature for the reaction
- 46. Changes in Concentration of Reactants and Products Increasing the concentration of the reactants creates a situation where Q is less than K; therefore, the system will adjust itself to produce more product so that Q will equal K, i.e., the equilibrium will shift toward the product.
- 47. Changes in Concentration of Reactants and Products Decreasing the concentration of the reactants creates a situation where Q is greater than K; therefore, the system will adjust itself to produce more reactants so that Q will equal K, i.e., the equilibrium will shift toward the reactants.
- 48. An Example of the Effect of Concentration Change on an Equilibrium Reaction If at 25oC, the equilibrium concentrations of n-butane and Isobutane are respectively 0.500 M and 1.25 M, what would be the equilibrium concentrations if 1.50 mol of n-butane is added to the equilibrium mixture?
- 49. 1.75 M - x 1.25 M + x C H C H C H C H ( C H ) C H C H 3 2 2 3 3 2 3
- 51. Changing the Volume If the number of moles of products equals the number of moles of reactants, then a change in volume of the reaction vessel will have no affect on the equilibrium. If the number of moles of product is greater than the number of moles of reactants, then an increase in volume of the reaction vessel will shift the equilibrium toward the formation of product. A decrease in the volume will shift the equilibrium toward the reactants.
- 52. Changing the Volume If the number of moles of reactant is greater than the number of moles of products, then an increase in the volume of the reaction vessel will shift the equilibrium toward the reactants. A decrease in volume will shift the equilibrium toward the products.
- 53. Effect of Pressure Change on a Gaseous Reaction If the number of moles of gaseous products equals the number of moles of gaseous reactants, then pressure changes have no effect on the equilibrium reaction. If the number of moles of gaseous products is greater than the number of moles of gaseous reactants, then an increase in pressure shifts the equilibrium toward the reactants. A decrease in pressure shifts the equilibrium toward the products.
- 54. Effect of Pressure Change on a Gaseous Reaction If the number of moles of gaseous reactants is greater than the number of moles of gaseous products, then an increase in pressure shifts the equilibrium toward the products. A decrease in pressure shifts the equilibrium toward the reactants.