HPLC[ HIGH PERPROMANCE LIQUID CHROMATOGRAPHY OR HIGH PRESSURE LIQUID CHROMATOGRAPHY, BRIEF HISTORY, DEFINITION, WHAT IS HPLC? USES OF HPLC, SEPARATION MECHANISM, CLASSIFICATION, ELUTION TEXHNIQUES, GRADIENT AND ISOCRATIC, NORMAL PHASE AND RP- HPLC , ST
- 1. HPLC Prof. Ravisankar Vignan Pharmacy college Valdlamudi Guntur Dist. Andhra Pradesh India. banuman35@gmail.com 00919059994000
- 2. Brief History and Definition Liquid chromatography was defined in the early 1900s by the work of the Russian botanist, Mikhail S. Tswett. His studies focused on separating compounds [leaf pigments], extracted from plants using a solvent, in a column packed with particles. Tswett filled an open glass column with particles. Two specific materials that he found useful were powdered chalk [calcium carbonate] and alumina. He poured his sample [solvent extract of homogenized plant leaves into the column and allowed it to pass into the particle bed.. The compounds that were more strongly attracted to the particles slowed down, while other compounds more strongly attracted to the solvent moved faster. This process can be described as follows: the compounds contained in the sample distribute, or partition differently between the moving solvent, called the mobile phase, and the particles, called the stationary phase. This causes each compound to move at a different speed, thus creating a separation of the compounds. Tswett coined the name chromatography [from the Greek words chroma, meaning color, and graph, meaning writing—literally, color writing] to describe his colorful experiment. Curiously, the Russian name Tswett means color. Tswett's Experiment The concept of the first HPLC was put forth by Kirkland and Huber b/n 1967 and 1969.
- 3. History of HPLC: Prior to the 1970's, few reliable chromatographic methods were commercially available to the laboratory scientist were carried out using a variety of techniques including open-column chromatography, paper chromatography, and thin-layer chromatography. However, these chromatographic techniques were inadequate for quantification of compounds and did not achieve sufficiently high resolution to distinguish between similar compounds. During this time, pressure liquid chromatography began to be used to decrease flow through time, thus reducing purification times of compounds being isolated by column chromatography. However, flow rates were inconsistent, and the question of whether it was better to have constant flow rate or constant pressure was debated. High pressure liquid chromatography was developed in the mid-1970's and quickly improved with the development of column packing materials and the additional convenience of on-line detectors. In the late 1970's, new methods including reverse phase liquid chromatography allowed for improved separation between very similar compounds.
- 4. • By the 1980's HPLC was commonly used for the separation of chemical compounds. New techniques improved separation, identification, purification and quantification far above those obtained using previous techniques. Computers and automation added to the convenience of HPLC. Additional column types giving better reproducibility were introduced and such terms as micro-column, affinity columns, and Fast HPLC began to immerge. • The past decade has seen a vast undertaking in the development of micro-columns, and other specialized columns. The usual diameter of micro-columns, or capillary columns, ranges from 3 µm to 200 µm. Fast HPLC utilizes a column that is shorter than the typical column. A Fast HPLC column is about 3 mm long and is packed with smaller particles. • Although HPLC is widely considered to be a technique mainly for biotechnological, biomedical, and biochemical research as well as for the pharmaceutical industry,in actual fact these fields currently comprise only about 50% of HPLC users. Currently HPLC is used in a variety of fields and industries including the cosmetics, energy, food, and environmental industries.
- 5. Introduction: HLC is basically a high improved form of column chromatography. Instead of solvent being allowed to drip through a column under gravity, it is Forced through under high pressure up to 400 atmospheres. That makes it much faster. In HPLC it is also allows you to use a very much smaller particle size (3-5µm)for column packing. Material which gives a much greater surface area for interactions b/n the stationary phase And the mobile phase it. This allows the better separation of the components of the complex mixture. This in turn Increase the elution by over 100 folds. The other major improvement over column chromatography concerns the Detection methods which can be used. These methods are highly automated and extremely sensitive. What Is UltraPerformance Liquid Chromatography (UPLC Technology)? In 2004, further advances in instrumentation and column technology were made to achieve very significant increases in resolution, speed, and sensitivity in liquid chromatography. Columns with smaller particles [1.7 micron]and instrumentation with specialized capabilities designed to deliver mobile phase at 15,000 psi [1,000 bar] were needed to achieve a new level of performance. A new system had to be holistically created to perform ultra-performance liquid chromatography, now known as UPLC technology.
- 11. H P igh ressure L iquid C hromatography Since high pressure is used when compared to classical chromatography Separation of the components from a mixture is achieved by pumping mobile phase at High pressure using appropriate displacement pumps or gas pressure. (Due to the small particle size (3-5 um).
- 12. H P igh erformance L iquid C hromatography More ever it is improved performance when compared to other conventional column Chromatographic techniques.
- 13. (advantages)
- 15. Determination Peak area (or height) is proportional to the concentration (or amount) of the component. The concentration of component A(caffeine) is determined by comparing the peak area with that of the standard caffeine peak. What is the concentration of component A? A B C Caffeine (1mg/ml) 5ul injection (5ug)
- 19. A B C Results obtained by HPLC Chromatogram containing three peaks Qualitative analysis (identification) and Quantitative analysis (determination) Can be performed using the information contained in the chromatogram Chromatography : Method Chromatogram : Results Chromatograph : Instrument
- 20. Separation Mechanism A B C time . CBA Column Packing material ↓ ↓ ↓ ↓ Mobile phase (solvent) C > B > A
- 22. CLASSIFICATION OF HPLC: BASED ON MODE OF RETENTION BASED ON OPERATING METHOD BASED ON MODE OF SEPARATION
- 23. BASED ON MODE OF RETENTION ADSORPTION PARTITION SIZE EXCLUSION AFFINITY ION EXCHANGE Liquid mobile phase and solid adsorbent Liquid mobile phase and ionic stationary phase Liquid mobile phase and liquid stationary phase Molecular modelling Lock & key
- 24. (A) uses charge, (B) uses pores, and (C) uses covalent bonds to create the differential affinities among the mixture components for the stationary phase.
- 27. BASED ON OPERATING METHOD ELUTION DISPLACEMENT ISOCRATIC GRADIENT FRONTAL
- 28. Based on elution Technique There are 2 types of elution : 1- Isocratic elution : fixed ratio of mobile phase components during the analysis. 2- Gradient elution (mobile phase programming) :the components of mobile phase ratio is changed through out the analysis for separation of components very close in polarity.
- 30. BASED ON MODE OF SEPARATION; MODE OF SEPARATION STATIONARY PHASE MOBILE PHASE NORMAL PHASE POLAR NON POLAR REVERSE PHASE NON POLAR POLAR
- 31. 1.Based on Modes of chromatography: There are 2modes (Normal phase mode and Reverse phase mode) depending on the polarity of the stationary and mobile phase. Polar- polar --- interaction (or) affinity is more. Nonpolar- Nonpolar– interaction (or) affinity is more. Polar- Nonpolar --- Interaction is less. Remember, "Like attracts like” in polarity Those with dissimilar polarity exhibit much weaker attraction, if any, and may even repel one another.
- 33. Normal Phase Mode HPLC or NP- HPLC or Normal phase HPLC: This is essentially just the same as you will already have read about in TLC or Column chromatography. Although it is described as “Normal” it is not most commonly used form of HPLC. In This separations of plant extracts, Tswett was successful using a polar stationary phase [chalk in a glass column; see Figure A] with a much less polar [non-polar] mobile phase. This classical mode of chromatography became known as normal phase. normal-phase chromatographic separation of our three-dye test mixture.,The stationary phase is polar and retains the polar yellow dye most strongly. The relatively non-polar blue dye is won in the retention competition by the mobile phase, a non-polar solvent, and elutes quickly. Since the blue dye is most like the mobile phase [both are non-polar], it moves faster. It is typical for normal-phase chromatography on silica that the mobile phase is 100% organic; no water is used. The commonly used columns for NP-HPLC include Cyano, amino and diol bonded phases As well as base silica or its derivatives. Stationary phase (Polar) Mobile phase (Non- Polar) Non polar material elutes first.
- 34. Normal Phase Chromatography Interaction : Adsorption Packing materials : Polar ex. Silica gel Silica-NH2 Silica-CN Silica-OH Mobile phase : Non-polar ex. n-Hex/CH2CL2 iso-Oct/IPA iso-Oct/AcOEt Sample : Fat-soluble Different polarity
- 35. Stationary phases for Normal-Phase chromatography: Common porous adsorbents such as silica & Alumina, that have polar hydroxyl groups on surface. In addition to porous adsorbents, a variety of polar bonded phases exist in some functional groups, such as cyano [-(CH2)3CΞN], diol [-(CH2)3OCH2CH(OH)CH2OH] Amino groups [-(CH2)n NH2, where n is 3 or 4],
- 36. Solvent Characteristics n-Hexane Mobile phase n-Heptane Mobile phase Isooctane Mobile phase 1-chlorobutane Large dipole Chloroform Proton donor Methylene chloride Large dipole Ethylene acetate Proton donor Tetrahydrofuron Proton acceptor Propylamine Proton acceptor Acetonitrile Dipole Mobile phases for Normal-phase chromatography:
- 37. Applications of Normal phase chromatography: Analysis of samples that are soluble in non polar solvents. Separation of isomers. Fat and water soluble vitamins ,Hydrocarbons and Pesticides have been separated using Hexane as mobile phase. Separation of saccharides in food and biological products.
- 39. CH3 CH2COOCH3 CH3 CH2COOCH3 Stationary phase (Non polar) Mobile phase (Polar) P0lar material elutes first.
- 40. 3. Separation mode Reversed Phase Chromatography Si Si O-Si-CH2(CH2)16CH3 CH3 CH3 O-Si-CH2(CH2)16CH3 CH3 CH3 O-Si-CH2(CH2)16CH3 CH3 CH3 CH3 CH3 O-Si-CH3 Silica-C18 Packing materials Commonly used packing materials are hydrocarbons having 18 carbon atoms (called the Octadecyl radical) which are chemically bonded to silica gel (Silica- ODS).Since the surface of the Silica- ODS is covered with hydrocarbon, the polarity of the packing material itself is very low. Today, because it is more reproducible and has broad applicability, reversed-phase chromatography is used for approximately 75% of all HPLC methods In this technique the silica is modified to make it non-polar by attaching long hydrocarbon Chain to its surface typically with either 8 or 18 carbon atoms in them.
- 41. Stationary phases for Reverse phase chromatography: Functional group chemically attached to a silica support- Bonded phase. Popular bonded phases; •Alkyl-C4H9,C8H17,C18H37 •Phenyl-C6H5
- 42. solvent Solvent polarity(P’) Water Dimethyl sulfoxide Ethylene glycol Acetonitrile Methanol Acetone Dioxane Ethanol Tetrahydrofuran 2-Propanol 10.2 7.2 6.9 5.8 5.1 5.1 4.8 4.3 4.0 3.9 Mobile phases for Reverse-phase chromatography:
- 43. Applications of Reverse phase chromatography: Pharmaceutical industry; steroids,vitamins,beta blockers Clinical laboratories; catecholamines Chemical industry; polymer additives Environmental pesticides and herbicides Food and beverage industy; sweetners and additives
- 44. of C Stationary phases Mobile phases Applications al phase C Bonded phase silica gel with functional groups such as Cyano [-(CH2)3 C=N] Diol [-(CH2)3 OCH2-CH-OH CH2OH Amino [-(CH2)2NH2] (n=3 or 4) n-Hexane, n-heptane, isooctane, 1-chlorobutane, CHCL3, CH2CL2, Ethyl acetate, THF, propylamine, acetonitrile and MeOH - Fat & oil soluble vitamins - Hydrocarbons - Pesticides - 8,9-oxide of avermectin B1 sed HPLC Bonded phase silica gel with functional groups (-C4 H8) – butyl (-C8 H17) – octyl (-C6 H5) – phenyl C18 – octadecyl Water, dimethyl sulfoxide, ethylene glycol, ACN, MeOH, acetone, dioxane, EtOH, THF & 2-propanol. - Most popular mode for the separation of low molecular weight neutral species that are soluble in H2O - Steroids - B Vitamins - β-blockers - Catecholamines - Polymer additives - Pesticides & herbicides - Carbohydrates - Sweeteners - Food additives - Aminoacids, peptides, nuclesedes, oligosaccharides xchange C Organic ion-exange resins containing exchange sites. The resin is composed of polystyrene- divinyl benzene Functional groups present in different ion exchange resins: Acids, Alkali, Buffers, Acetate buffer, Borate buffer Analyses of amino acids, nucleotides, (carbohydrates at PH >12) & proteins Inorganic anions, organic acids, inorganic cations etc. Fractionation of cations inorganic lanthanides peptides, amino acids, B.vitamins Strong Cation Exchange Resin: Sulfonic acids (-SO- 3H+ ) (sulfonated polystyrene) Weak Cation Exchange Resins: (polystyrene with functional groups) + Fractionation of cations inorganic lanthanides peptides, amino acids, B.vitamins Fractionation of cations Biochemical separations organic
- 45. GPC : Gel Permeation Chromatography GFC : Gel Filtration Chromatography Separation modes and features Mode Stationary phase Mobile phase Interaction Feature Normal phase Silica gel Organic solvent Adsorption Fat-soluble chromatography (n-Hexane/IPE) Reversed phase Silica-ODS MeOH/Water Hydrophobic Most widely used chromatography (Silica-C18) Size exclusion Chromatography Non-aqueous (GPC) Porous Polymer Organic solvent (THF) Gel permeation Molecular weight distribution Aqueous (GFC) Aqueous porous Polymer Buffer solution Gel permeation Protein Separation Ion exchange Ion exchange gel Buffer solution Ion exchange Separation of Chromatography ionic substances Affinity Packing with ligand Buffer solution Affinity Purification of Chromatography enzymes and proteins
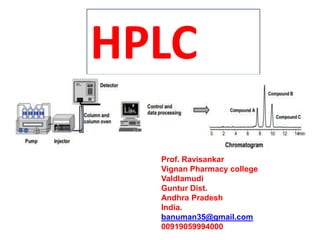
- 46. High performance (or high pressure) liquid chromatography (HPLC) is a separation technique in which a sample mixture is introduced into a stream of solvent (the mobile phase) which then flows over a surface (the stationary phase) consisting of either spherical particles or a film coated onto such particles. Separation results from differences between sample components in their relative affinity for the stationary and mobile phases
- 47. o Solvent reservoirs (mobile o Phase reservoirs) o Pump o Injector o Column o Detector o Recorder o Pulse Damper o Degasser o Column Heater Typical HPLC system consists of followings
- 48. A Modern HPLC instrument contains Glass or stainless steel reservoirs of capacity of 500mL,1000ml,2000ml,5000ml reservoir kit. for storing the mobile phase. Provisions are included to remove dissolved gases and dust from the liquid. Mobile phase reservoirs and Solvent pretreatment systems: 1000 ml HPLC Solvent Reservoir Kit. Comes with 1L solvent reservoir, cap and teflon insert for 1/8" OD tubing, 1 meter 1/8" OD PTFE tubing and 10 um 316 stainless steel frit. $60.00
- 49. •The reservoir and its attachment to the pump should be made of materials that will not contaminate the mobile phase: Teflon, glass, or stainless steel. •The vessel should have some sort of cap to prevent particulate matter from contaminating the mobile phase. •If you are using a solvent bottle as a reservoir, the top of the bottle can be wrapped in aluminium foil to keep dust out or the bottle cap can be drilled to allow inserting the inlet line through the cap. •Don't close the bottle too tightly or removal of mobile phase by the pump will create a vacuum. This prevents mobile phase from flowing the pump, creating a "vapour lock" within the pump. •The material chosen for constructing mobile phase reservoirs should not be reactive towards the mobile phase. Examples: Acidic or alkaline solvents can not be stored in metallic reservoirs. Non-polar solvents (chloroform) cannot be stored in polymeric reservoirs.
- 50. It is important that particulate matter be kept out of the mobile phase since particulates can damage the pump and injector as well as plug the column. Mobile phases are often filtered before adding them to the reservoir. In addition, a 10- micron frit or inlet filter should be connected to the end of the inlet line that dips into the reservoir. This inlet frit serves more than one purpose. Besides providing extra protection against particulates entering the pump, the inlet filter serves to hold the inlet line at the bottom of the reservoir. The stiffness of the inlet line tends to allow this Teflon tubing to creep out of the reservoir, preventing use of all the mobile phase unless an inlet filter is used to weigh down the end of the tubing. For this reason, inlet filters are often called "sinkers". Sinker" frits help keep the inlet line submerged and provide a final line of defense against particulate contamination. The sinker frit is not a substitute for filtering the mobile phase. The typical pore size used in a sinker frit is on the order of 5 - 10 microns frits of smaller pore size are too likely to plug. In general, the mobile phase should be filtered through a 0.3 to 0.5-micron frit after the solvents and buffers are mixed in order to remove particulate matter. The sinker frit protects against the occasional larger dust particle encountered after the mobile phase has been filtered. frit or inlet filter
- 51. Filter systems: Micro filters are (1-5µm) are placed before the solvent reservoirs in modern HPLC Instruments for removing the particulate matter as well as dust under vacuum. purity of the solvents is utmost important because the particulate matter Even in trace amounts interferes with the detector performance.
- 52. SOLVENT DEGASSING IN HPLC: Solvent De-gassing External vacuum Degassing Helium Degassing On-line Degassing Source: Phyllis R. Brown Kingston, Rhode island Pg. No:78-79
- 53. Sparging: It is important to remove any dissolved gas from the mobile phase before it is pumped into the system. H PLC analysis may contain gases such as oxygen that are non-visible to our eyes. When gas is present in the eluent, this is detected as a noise and causes unstable baseline. This prevents bubble formation that might damage the column and degrade system performance. Most systems provide a means to pass helium through the solvent reservoirs in a process called “sparging”. This strips out any dissolved gas, but helium’s low solubility and inert nature prevent it from being a problem itself. The image above shows this procedure being performed on all four reservoirs.
- 54. Generally used method includes sparging (bubbling of inert gas), use of aspirator, distillation system, and/or heating and stirring. However, the method is not convenient and also when the solvent is left for a certain time period (e.g., during the long analysis), gas will dissolve back gradually. Degasser uses special polymer membrane tubing to remove gases. The numerous very small pores on the surface of the polymer tube allow the air to go through while preventing any liquid to go through the pore. By placing this tubing under low pressure container, it created pressure differences inside and outside the tubing (higher inside the tubing). This difference let the dissolved gas to move through the pores and remove the gas. Compared to classical batch type degassing, the degasser can be used on-line, it is more convenient and efficient. Many of new HPLC unit system contain a degasser.
- 55. Solvents used : The mobile phase is used to transport the sample components through the column over the surface of the stationary phase. The exact choice of mobile phase depends on the nature of both the sample components to be separated and the column stationary phase. organic compounds are commonly separated using water-methanol or water- acetonitrile mixtures. For Normal phase HPLC ( or) NP_HPLC (or) Straight -phase HPLC. There are 4 commonly used solvents. In HPLC pure analytical grade Organic solvents are used (known as HPLC solvents) It has been duly established That “the stronger the solvent Or solvent mixture, the more Quickly it will elute an analyte (i.e., an organic compound) from A specific HPLC column)
- 56. Solvent choice: The mobile phase is used to transport the sample components through the column over the surface of the stationary phase . In normal phasehplc, it would typically be a non-polar solvent or mixture of solvents, such as hexane, cyclohexane, toluene, or dichloromethane. In reverse phase hplc, it is typically either one of, or a mixture of two or more of, the following: water, an aqueous buffer, methanol, acetonitrile, or tetrahydrofuran (THF). The exact choice of mobile phase depends on the nature of both the sample components to be separated and the column stationary phase. Ion chromatography, for example, often uses an aqueous buffer, while organic compounds are commonly separated using water-methanol or water-acetonitrile mixtures.
- 57. HPLC Pump Classification based on the Flow Rate Microbore Standard bore Preparative (1-250µL/min) (10µL/min—100 µL/min) (>10 mL/min) SOLVENT DELIVERY SYSTEM (PUMPS):
- 58. Classification According to mechanism of Eluent Displacement Solvent delivery system Syringe pumps Reciprocating-piston pumps Single head Multi head SOLVENT DELIVERY SYSTEMS(PUMPS)
- 59. HPLC pumps can be single, binary, or ternary, meaning that the can make use of one, two, or more different solvent reservoirs. (Most ternary pumps actually have four solvent reservoirs.) The image on the right shows a fairly typical ternary pump, which can be controlled viaeither the front panel or computer control. The key parts of such a pump system are the proportionating valve, the mixing chamber, the purge valve, and the actual pump mechanism.
- 60. Solvent mixing: The proportionating valve (lower-front of image) allows the desired volume ratio of the different solvents to be drawn from the reservoirs. For example, if reservoir A contained water and B methanol, the following pump settings would give a 50:50 water:methanol mixture – A=50%, B=50%, C=0%, D=0%. To ensure a uniform composition, a mixing chamber (middle-right of image) is used. This particular pump uses a reciprocating piston (upper-centre of image) to actually deliver the mobile phase mixture at the required flow rate.
- 61. Pump assembly This view shows the pump dis assembled. The glass rod in the centre of the image is the piston; the pump chamber has been removed. The solenoid valve to the upper-left of the piston is one of the proportioning valves. Routine maintenance requires that the seals preventing leaks around the piston be replaced periodically This view shows the pump body removed from the piston assembly. The piston assembly connects to the large collar on the left of the unit. The mobile phase enters through the top-right connector and leaves through the bottom-left connector. The nuts adjacent to these on the upper and lower sides actually retain the check valves, which are essential to the working of the pump; they basically determine the direction through the pump taken by the mobile phase. Check valves:
- 62. Reciprocating piston pumps do not work if there is air in the lines, which can be the case after changing a solvent reservoir, or leaving the pump off. Opening the purge valve allows the contents of the lines to be flushed out without the contents entering the injector or column. It’s important to remember to close the purge valve before trying to perform an experiment! Purge valve:
- 63. Classification According To Materials Of Construction Solvent Delivery system Metallic Non-metallic Steel Titanium PEEK Teflon Ceramic SOLVENT DELIVERY SYSTEMS (PUMPS)
- 64. Pumps In the earlier state of HPLC development, the pump was the most important part of the system. The development of HPLC can be said that it was a development of pump system. However, nowadays, the high pressure generation is a “standard” requirement and what is more concerned nowadays is to be able to provide a consistent pressure at any condition, Most pumps used in current HPLC systems generate the flow by back-and-forth motion of a motor-driven piston (reciprocating pumps). The pump is a heart of the instrument. It can deliver the solvent through the chromatograph with a pressure not less than 3000 Psig and 6000 Psig.
- 65. SYRINGE PUMPS: Syringe Pumps: These are also called as positive displacement pumps. These are popular as pulse less solvent delivery systems in HPLC. The syringe pump contains a large barrel syringe with the plunger connected to a motor. As the plunger moves forward, it drives the eluent through the chromatograph with a pulse less flow; the main advantage of syringe pumps is that it generates stable flow.
- 66. WORKING OF A RECIPROCATING PUMP:
- 69. Isocratic pumps versus Gradient pumping systems: Isocratic Pumping Systems Gradient Pumping Systems: An Elution with single solvent or mixture of solvent of constant composition is termed as Isocratic elution. Two or more solvents are used for the elution of analytes is termed as Gradient Elution.
- 70. High pressure mixing: In high pressure mixing the eluents are blended on the injector side, or high pressure side of the pump. For this purpose two isocratic pumps are required. High pressure mixing is widely used in HPLC-MS and capillary-LC. Low pressure mixing: Low pressure mixing is a common design for modern gradient HPLC systems. In this the solvents are blended at atmospheric pressure. By means of a small Teflon block with 3 or 4 proportioning values, a mixture of any combination of the four solvents is delivered to the low pressure intake side of the pump.
- 71. Based on the elution technique there are 2 types of HPLC techniques: There are 2 types of elution : 1- Isocratic elution : fixed ratio of mobile phase components during the analysis. 2- Gradient elution (mobile phase programming) :the components of mobile phase ratio is changed through out the analysis for separation of components very close in polarity. 1. Isocratic elution(separation) technique: Isocratic elution is where the composition of the mobile phase is unchanged during the entire elution process. a. In this elution technique in which the components of mobile phase are maintained constant throughout the process of separation is known as isocratic elution. b. In this technique the mobile phase may contains single or two or more solvents of similar polarity to elute the sample through the column. Examples of mobile phases for isocratic elution are Chloroform,Benzene:petether(1:1) In this technique changing the length/internal diameter of the column, the elution order of the peaks remains same i.e., selectivity does not change. In gradient elution any change in the dimensions of the column immediately affects the elution order. An isocratic elution cannot separate a complex mixture with adequate resolution and good detectability. Hence it is used only in the following cases… 1. When sample mixture contains less than 10 weakly retained components. 2. When gradient base line negatively effects trace analysis.
- 72. Advantages of isocratic elution technique: 1. Components of mobile phase remains constant through the process. Hence easy to handle and does not require continuous monitoring. 2. Any change in column dimensions does not change the elution order (peaks are of same order. Disadvantages: 1. Late eluting peaks are flat and broad. 2. Takes longer time than gradient elution for complex mixtures.
- 73. HPLC analysis is a prominent analytical tool in pharmaceutical laboratories. Once a method for the separation is developed, students can conduct both qualitative and quantitative analysis of an analgesic mixture. These experiments indicated that the analgesic compounds could be divided into two groups of polar and non-polar molecules. a) Isocratic elution of polar analgesics:he group of polar molecules included Aspirin, Acetaminophen and caffeine which could be eluted on an octylsiloxane, C-8, column. Furthermore, the mobile phase was composed of 67% H 2O, 3% 5 mM KH 2PO 4 (pH 6.1) and 30% methanol (volume by volume). Note that the presence of the potassium phosphate buffer was necessary in order to obtain reproducible results, particularly with respect to each component’s retention time. The optimized experimental conditions were as follows:
- 74. A multicomponent mixture of six analgesic drugs commonly found in over-the-counter pain-relieving formulations: Aspirin, Acetaminophen, caffeine, Naproxen, Ibuprofen and salicylamide.
- 75. Once a method for the separation is developed, students can conduct both qualitative and quantitative analysis of an analgesic mixture In order to develop the gradient elution analysis, it was first necessary to conduct many preliminary trial-and-error runs. These experiments indicated that the analgesic compounds could be divided into two groups of polar and non-polar molecules..
- 76. a) Isocratic elution of polar analgesics The group of polar molecules included Aspirin, Acetaminophen and caffeine which could be eluted on an octylsiloxane, C-8, column. Furthermore, the mobile phase was composed of 67% H 2O, 3% 5 mM KH 2PO 4 (pH 6.1) and 30% methanol (volume by volume). Note that the presence of the potassium phosphate buffer was necessary in order to obtain reproducible results, particularly with respect to each component’s retention time. The optimized experimental conditions were as follows:
- 77. The following HPLC chromatogram was obtained by elution of a mixture of the three polar analgesics according to the conditions specified above
- 78. b) Isocratic elution of non-polar analgesics The remaining compounds, namely, Naproxen, salicylamide, and Ibuprofen are relatively non-polar and therefore could be eluted from a C-8 column using a mobile phase (solvent) that was predominantly made up of methanol. The optimized conditions for the separation of the non-polar analgesic group were as follow
- 79. The following HPLC chromatogram was obtained by elution of a mixture of non- polar analgesics Note that under these conditions, it was possible to resolve Naproxen and Ibuprofen only. When a mixture of all three components was chromatographed, considerable overlap between Ibuprofen and salicylamide took place. The latter was probably due to the relatively polar nature of the octylsiloxane, C-
- 80. Gradient elution of polar and non-polar analgesics According to the preceding isocratic elution experiments, the polar analgesic compounds Aspirin, Acetaminophen and caffeine required a relatively polar solvent. The mobile phase was made up of 67% H 2O, 3% 5 mM KH 2PO 4 (pH 6.1) and 30% methanol (volume by volume). By contrast, the non-polar analgesics Naproxen and Ibuprofen required a non-polar solvent. The mobile phase was made up of 7% H 2O, 3% 5 mM KH 2PO 4 (pH 6.1) and 90% methanol (volume by volume). Because of the singular discontinuity in the polarity, the solvent ramp to resolve the analgesic mixture involved two time-dependent steps of decreasing polarity: in step 1 the mobile phase contained 67% H 2O, 3% 5 mM KH 2PO 4 (pH 6.1) and 30% methanol(volume by volume), for a period of two minutes. In step 2 the mobile phase was made up of 100% methyl alcohol. Thus, in the first transient, the mobile phase is predominantly aqueous and therefore highly polar -- it elutes the polar analgesics. Conversely, in the second transient, the solvent is predominantly methanol which is relatively non-polar -- it elutes the non-polar analgesics.
- 81. The gradient method operated as follows. When the sample was first loaded onto the column, the mobile phase was predominantly water. Consequently, the more polar Aspirin, Acetaminophen and caffeine traversed the stationary phase. The relatively non- polar Naproxen and Ibuprofen remained tightly adsorbed at the column’s gate. After two minutes, as shown by the vertical white marker, switching to absolute methanol decreased the polarity of the mobile phase. As a result, the non-polar analgesics, Naproxen and Ibuprofen, were successfully dislodged from the non-polar matrix and eluted by the solvent. A mixture containing Aspirin, Acetaminophen, caffeine, Naproxen and Ibuprofen was then analysed under gradient elution conditions. As the chromatogram below shows, all five analgesics were successfully resolved.
- 82. Isocratic elution for simple mixtures. Gradient elution for complex mixture. Isocratic and Gradient(techniques) HPLC System Operation Two basic elution Techniques (modes) are used in HPLC. The first is called isocratic elution. In this technique (mode), the mobile phase, either a pure solvent or a mixture, remains the same throughout the run. Isocratic HPLC System
- 83. Gradient elution Technique: Gradient elution is where the solvent strength of the moblie phase in increased during the elution starting with a solvent of relatively low solvent strength. Steady changes of the mobile phase composition during the chromatographic run is called gradient elution. It may be considered as an analogy to the temperature programming in gas chromatography. The main purpose of gradient elution is to move strongly retained components of the mixture faster, but having the least retained component well resolved. Gradient HPLC also known as solvent programming is a technique employed in HPLC. Starting with the low content of the organic component in the eluent we allow the least retained components to be separated. Strongly retained components will sit on the adsorbent surface on the top of the column, or will move very slowly. When we start to increase an amount of organic component in the eluent (acetonitrile) then strongly retained components will move faster and faster, because of the steady increase of the competition for the adsorption sites. For example in a HPLC technique mobile phase as a mixture of A and B components. The initial concentration of the component A(organic solvent) is say 10%,then after A time period of about 20 min. the concentration is steadily increased to about 90%. The other component B, is water which is regarded as the weak solvent that allows the Elution of solutes very slowly, while the organic solvent is considered as the strong solvent that rapidly elutes analytes from the column. The commonly employed organic solvents include methonol,acetonitrile,isopropanol,THF etc.
- 84. Performance of the gradient elution is strongly dependent on the instrumentation. Two main points the chromatographer needs to know about his instrument: How large the volume between the component mixing point and column inlet is. For the low-pressure gradient systems this volume usually correspond to the pump volume, and about 2 - 3 ml. How well does the system mix eluent components. If the system does not mix components well then it will supply for the certain time one component then another and so on. Chromatographic performance of such system will be very low especially for the least retained components. Advantages: -The technique increases the efficiency of columns -Complex sample mixtures with wide retention range can be separated. -Samples which contain a unknown composition can be screened. -Retention of late eluting components is decreased. Hence they elute faster and give narrower peaks. Disadvantages: -Requires continuous monitoring. -Maintenance cost is more. -The additional mixing of liquids causes a delay in the formation of gradient. -Any change in column dimension changes the elution order (peaks are of different order)
- 85. The second type is called gradient elution technique, wherein, as its name implies, the mobile phase composition changes during the separation. This mode is useful for samples that contain compounds that span a wide range of chromatographic polarity. As the separation proceeds, the elution strength of the mobile phase is increased to elute the more strongly retained sample components. High-Pressure-Gradient System
- 86. Column heater The LC separation is often largely influenced by the column temperature . In order to obtain repeatable results, it is important to keep the consistent temperature conditions. Also for some analysis, such as sugar and organic acid, better resolutions can be obtained at elevated temperature (50~80 °C). Thus columns are generally kept inside the column oven (column heater).
- 87. The function of injector in HPLC is to introduce the sample in the flowing solvent so that it may be carried to the column. Injector may be operated either manually or automatically Manual has two different modes for injecting samples Sample Injector Device: 1.Septum mode injection 2.Valve injection
- 88. 1. Septum injection: • This allows injecting sample into pressurized solvent stream using a self-elastic septum with a micro syringe • It is the simplest form of injector in the septum device (similar to one used in GC). Greatest Drawback: Limited to max operating pressure of 1500 psi. Therefore, not widely used in HPLC anymore.
- 89. Widely used in HPLC, Allows reproducible introduction of sample into the pressurized M.P without interruption of flow even at high temperature. Six-port Valve Type Injector One of the simplest and most common valve injector in HPLC is the “six-port valco”(or Rheodyne model) injector. Inject valve has two position: (a) Load (b) Inject 2. Valve-Type Injection in HPLC:
- 94. COLUMNS FOR HPLC; Empty Stainless steel HPLC column hardware come with the threaded tube, two 2um frits, and two stainless steel end fitting nuts. 2.1, 3.0, 4.0, 4.6, 7.8, 10, 22, and 30mmI.D. and 50mm,100mm.150mm, 250mm and 300mm length
- 95. . For normal phase, SIL or DIOL is usually the best choice while C18, C8, C4 or Phenyl is the best choice for reversed phase applications
- 97. Stationary phases in column: Silica gel Alumina Zirconium Carbon Hydroxyapatite. Polymeric adsorbents such as polystyrene-divinyl benzene. Packed with 3-10µm particles. Resin based packings such as Polystyrene-divinylbenzene & Acrylic based polymers are used in HPLC columns. :
- 98. COLUMNS FOR HPLC: A column is a heart of the chromatograph for separating a mixture into components. Column Packing material container Nature of particle Particle size Length Diameter Material
- 99. Pore size: Porous microparticles are most common particles used in HPLC. Particles with small pores exhibit a high surface area & greater retention. Pore size Porous particle Pellicular particle Porous microparticle Stationary phases in column:
- 100. Particle size: Small particle sizes result in high efficiencies with increased back- pressure, results decreased column permeability. Porous particle ranges from ~20-40µm. Porous micro particles are chosen for high efficiency, ranges from 3-10µm Internal diameter of column: Internal diameter will effect the sample load, peak dilution, flow rate. Larger the diameter, greater the sampling load, higher the flow rate. Internal diameter for analytical columns ranges from 2-5mm Stationary phases in column :
- 101. Column length: Effects both efficiency & speed of separation. Column efficiency tends to increase with column length. Longer columns tends to longer time analysis. Ranges from 30-300mm in length. Construction materials of column: 316stainless steel for rigidity & mechanical strength at high pressures. Glass, Teflon, & PEEK Columns are used for aggressive mobile phases, such as hydrochloric acid, or with solutes such as proteins may adsorb to stainless steel. Polymeric columns for ion-exchange packings, glass for protein separation
- 102. One common stationary phase is a silica which has been treated with RMe2SiCl, where R is a straight chain alkyl group such as C18H37 or C8H17. With these stationary phases, retention time is longer for molecules which are more non- polar, while polar molecules elute more readily.
- 103. SiliaChrom AQ C18 columns use a new generation adsorbent based on high purity spherical silica and unique bonding technology. SiliaChrom AQ C18 is a slightly polar C18 column, and is the most versatile reversed phase column offered by SiliCycle for highly aqueous mobile phases. SiliaChrom AQ C18 is considered as the most universal HPLC column for any type of analytes (acidic, basic and neutral). Furthermore, SiliaChromAQ C18 is applicable to a wide range of sample types: plasma, urine, drug formulation, food extraction, forensic, clinical and bioanalytical. Main Characteristics •Exceptional stability at pH 1,5 to 9,0 •Inertness for acidic and basic analytes •Compatible from 100% aqueous mobile phase to 100% organic •Rapid equilibration •Reduced need for mobile phase modifiers •Partially endcapped
- 106. Reversed-Phase Column 1.1 ODS column The packing material used for reversed-phase column is often made of silica gel modified with functional grouphe most often used functional group is octadecyl. Octadecyl functional group is a straight carbon chain of 18. Its molecular structure is shown below. . Ideally, entire surface of ODS gel is modified with C18 functional group; however there will be remaining spaces that are not modified. Those part are called “residual silanol” and the presence of residual silanols can influence the separation. Often “end capping” is applied to the gels to immobilize the residual silanol. Almost all ODS columns nowadays are end-capped, however depending on the type of analyte, presence of silanol may provide better separation results.
- 107. Other Silica-Based Columns ODS is most popularly used RP column, however since C18 is a long chain, it may retain compounds too much and consequently results in long analysis time. Referring to our story, this maybe a case that promotion was “too effective” making the customer stay at the bar forever, which is not a good management for the bar. So in those cases, it is better to use functional group with shorter chain, such as C8, C4, and C3. C8:Octyl functional group -CH2CH2CH2CH2CH2CH2CH2CH3 C4:Butyl functional group -CH2CH2CH2CH3 C3:Trimethyl functional group -CH2CH2CH3 Also there are silica gels modified with phenyl and cyanopropyl functional groups. The most popular Shodex TM polymer column is ODP series. As you may guess, the name of ODP came from OctaDecyl Polymer. We believe it is easy to remember, as S (silica) in ODS was simply replaced with P (polymer) Polymer-Based Columns Long column life In general, polymer gel is chemically more stable than silica gel and deteriorates slow
- 108. •Despite that the column life of silica column is about 3 months whereas it is not surprising to see a polymer column provides stable analysis over 1 year. •Good repeatability •Usability under alkaline conditions Silica column cannot be used under alkaline conditions where polymer column can. Some basic samples (often pharmaceutical compounds) require alkaline mobile phase to obtain good separations, thus polymer columns are suitable for such analysis. Resolution and price General consensus is that polymer columns compared to silica columns are more expensive and provide less resolution. Therefore even though polymer columns have several advantages over silica columns, former is less often used. The price of the polymer column is becoming lower as the technology has been improved. Also considering the longer column life, from a long term point of view, polymer column is not extremely more expensive than silica columns. The biggest concern will be the resolution. However, again the performance of newer polymer column has been improved and is becoming very comparable to silica columns The most popular Shodex TM polymer column is ODP series. As you may guess, the name of ODP came from OctaDecyl Polymer. We believe it is easy to remember, as S (silica) in ODS was simply replaced with P (polymer). The next popular polymer-based RP column is DE-413. The packing gel is made of polymethacrylate.
- 109. Normal-Phase Column Silica gel without modification has large polarity, but when C18 functional group is modified, the polarity becomes small. The larger polarity of silica gel is due to these oxygen and hydrogen; the composition of silica is SiO2, but it has hydroxide group (oxygen + hydrogen) on its surface. RP mode uses gel with small polarity (e.g., ODS) and mobile phase with large polarity (e.g., water, acetonitrile). Normal-phase mode uses gel with large polarity (e.g. silica) and mobile phase with small polarity (e.g. hexane, chloroform). Instead of using the word small or large polarity, it is also common to use words, hydrophilic or hydrophobic. Something easy to dissolve in water (i.e., large polarity) is called hydrophilic and something easy to dissolve in oil (i.e., smal polarity) is called hydrophobic. At the very early stage of HPLC development, silica gel without any functional group was only used. Thus, historically normal-phase mode was developed first and so named “Normal”. Then the separation mode which uses opposite separation theory to normal phase was developed and named “Reversed”. RP mode is much more popularly used than normal phase nowadays, but we cannot change the historical background, and thus they are still called normal and reversed-phase modes.
- 110. The matrix of the primary silica gel particle consists of a core of silicon atoms joined together with oxygen atoms by siloxane bonds (silicon-oxygen-silicon bonds). On the surface of each primary particle some residual, uncondensed hydroxyl groups from the original polymeric silicic acid remain. These residual hydroxyl groups confer upon silica gel its polar properties. These hydroxyl groups react with the silane reagents to form bonded phases There are three types of hydroxyl group. The first is a single hydroxyl group attached to a silicon atom which has three siloxane bonds joining it to the gel matrix. The second is one of two hydroxyl groups attached to the same silicon atom which, in turn, is joined to the matrix by only twosiloxane bonds. These twin hydroxyl groups are called Geminal hydroxyl groups. The third is one of three hydroxyl groups attached to a silicon atom which is now only joined to the silica matrix by only a single siloxane bond. An example of each type of hydroxyl bond is …. adsorption could also take place the surface of siloxane bonds as well.
- 111. Silica gel is manufactured by releasing silicic acid from a strong solution of sodium silicate by hydrochloric acid. (Sodium silicate is prepared by heating sand at a high temperature in contact with caustic soda or sodium carbonate). Initially, silicic acid is released, Na2SiO3 +H2O + 2HCl = Si(OH)4 + 2NaCl and then the free acid quickly starts to condense with itself with the elimination of water to form dimers, trimers and eventually polymeric silicic acid. The polymer grows, initially forming polymer aggregates and then polymer spheres, a few Angstrom in diameter. Thesepolymeric spheres are called primary silica particles. These primary particles continue to grow until, at a particular size, the surface silanol groups on adjacent primary polymer particles, condense with the elimination of water. This condensation causes the primary particles to adhere to one another and at this stage the solution begins to gel. During this process, the primary particles of silica gel will have diameters ranging from a few Angstrom to many thousands of Angstrom depending on the conditions of formation.
- 112. his unique ultra-solvent-resistant computer- printable label range for HPLC columns is designed to permanently adhere to all surfaces and withstand aggressive HPLC cleaning agents. when exposed to methanol, ethanol, acetone, IPA, and THF, providing lab managers with the perfect HPLC identification solution. variable data (batch numbers, barcodes, etc
- 113. PRP Polymeric Reversed Phase Poly(styrene-divinylbenzene) HPLC Columns are used in quality assurance as well as research and development laboratories because they last significantly longer than silica-based reversed phase columns. This is particularly true for difficult analyses like: high pH samples (pH 8-13); labile or reactive samples (irreversible adsorption); high aqueous purifications (80-100% water); and separations with ion pairing reagents. HxSil silica-based columns are ideal for applications where a higher efficiency reversed phase C8 or C18 column is needed. Hamilton PRP-1 reversed phase columns Polymeric columns for general separations. •pH Stable from 1 to 13 •Better sample recovery than silica based columns. •Excellent durability (stable to any concentration of water or organic solvent). Product Features •Four particle sizes: 5, 7, 10 and 12- 20 µm. •Ten column internal diameters: 1.0 to 101.6 mm. •Two column materials: 316 stainless steel and PEEK
- 114. APPLICATIONS of PRP columns (polymeric Reverse phase columns Peptide Standards Resveratrol Folic Acid Pregnenolone Biotin, Folic Acid, Vitamin B12 Coenzyme Q10 Ginkgo Biloba Soy Isoflavins Fat Soluble Vitamins Phenobarbital, Scopolamine, Hyoscyamine, Ergotamine Pregnenolone, progesterone Hormones Erythromycins Pyridinoline Diphenylguanidine separations up to 9 times faster if required
- 115. Special fitting style guard column for protect main column! For 4.6mmI.D. HPLC column. Features ・Special fitting style guard column ・Easy hand tight connection Guard Column 3mm (Cartridge type)
- 117. End-Capping A reversed-phase HPLC column that is end-capped has gone through a secondary bonding step to cover unreacted silanols on the silica surface. End-capped packing materials eliminate unpredictable secondary interactions. Basic analytes tend to produce asymmetric tailed peaks on non end-capped columns, requiring the addition of modifiers to the mobile phase. Non end-capped materials exhibit different selectivity than end-capped columns. This selectivity difference can enhance separations of polar analytes by controlling the secondary silanol interactions. A column is said to be endcapped when a small silylating agent, such as trimethylchlorosilane, is used to bond residual silanol groups on a packing surface. It is most often used with reversed-phase packings and may cut down on undesirable adsorption of basic or ionic compounds.
- 118. what is the specific advantage in using endcapped columns against non endcapped one? Because of steric hindrance and other factors, it is not possible to react all of the silanols with C8 or C18 alkyl groups. Because of this, after the initial bonding, additional bondings are sometimes performed, attaching smaller alkyl groups, quite often propyl or isopropyl groups, to the residual silanols. There is no endcapping process that is complete. There are always unreacted silanols. The resulting stationary phase, having fewer residual silanols, will display fewer secondary interactions characteristic of silanols than a non- endcapped stationary phase. The result is less peak broadening and reduced tailing -- especially with organic acids and bases. The common endcapping group is a trimethylsilyl group
- 119. The silica is first dried at 250oC to remove the majority of the strongly bound water from the surfacehe silica is suspended in toluene that is contained in a flask fitted with a reflux condenser and then treated with an excess of dimethylchlorsilane (all the adsorbed water on the silica gel surface my not have been completely removed and the excess reagent will scavenge any residual water) The mixture is refluxed for about three hours and finally any remaining reagent or hydrochloric acid is The surface is now covered with a layer of dimethylsilane groups. Squalene is then added to the suspension, accompanied by the catalyst chlorplatinic acid, and the mixture is next refluxed for a further 6 hours. Squalene contains six double bonds along its chain, each double bond being separated by two methylene groups and thus, in the presence of the catalyst, any double bond coming in contact with the silane hydrogen on the silica surface will open and attach the squalene chain to that silyl group. reacted with trimethylchlorsilane to end cap the final methyloctylmonohydroxysilane groups that were generated on the surface
- 120. An end-capped C8 sorbent that offers moderate hydrophobic retention with negligible secondary polar interactions from active silanol groups. Description: Octadecyl (non end-capped) functionalized silica, manufactured using trifunctional silane. Average Particle Size: 50 µm (irregular shaped particles) Nominal Porosity: 60 Å Applications: Matrix – Aqueous Analytes – Wide range polarity Retention mechanism – Non-polar [polar, cation exchange] Comments: Strong non-polar (hydrophobic) phase. Non end-capped to provide additional silanol interactions.
