Biotransformation
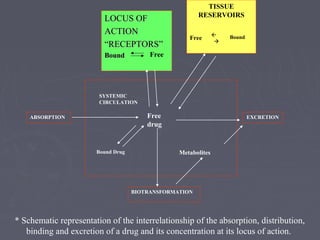
- 1. LOCUS OFLOCUS OF ACTIONACTION ““RECEPTORS”RECEPTORS” BoundBound TISSUE RESERVOIRS Free Bound SYSTEMIC CIRCULATION Free drug ABSORPTION EXCRETION BIOTRANSFORMATION Bound Drug Metabolites Free * Schematic representation of the interrelationship of the absorption, distribution, binding and excretion of a drug and its concentration at its locus of action.
- 3. BIOTRANSFORMATIONBIOTRANSFORMATION ►DefinitionDefinition ►ObjectivesObjectives ►SitesSites ►TypesTypes ►Biochemical ReactionsBiochemical Reactions ►Pattern of Biochemical ReactionsPattern of Biochemical Reactions ►Factors Affecting BiotransformationFactors Affecting Biotransformation
- 4. DEFINITION -DEFINITION - BIOTRANSFORMATIONBIOTRANSFORMATION BIO derived from GREEK Word BIOS meaning lifeBIO derived from GREEK Word BIOS meaning life Transformation meaning alteration /Transformation meaning alteration / changechange
- 5. AIMS AND OBJECTIVESAIMS AND OBJECTIVES The mechanisms involved in theThe mechanisms involved in the biotransformation / metabolism of drugs arebiotransformation / metabolism of drugs are discussed.discussed. Biotransformation causes: -Biotransformation causes: - 1.1. Conversion ofConversion of PRODRUGPRODRUG toto ACTIVEACTIVE DRUGDRUG 2.2. Conversion ofConversion of DrugDrug toto Inactive FormInactive Form 3.3. Conversion of aConversion of a DrugDrug to ato a ToxicToxic MetaboliteMetabolite
- 6. AIMS AND OBJECTIVESAIMS AND OBJECTIVES A clinician must be well informed about theseA clinician must be well informed about these processes to treat the patients without exposingprocesses to treat the patients without exposing them to the harmful effects of drugs.them to the harmful effects of drugs.
- 7. BIOTRANSFORMATIONBIOTRANSFORMATION Drugs are eliminated by biotransformation andDrugs are eliminated by biotransformation and excretion intoexcretion into URINEURINE oror BILEBILE.. Major site of drugs biotransformation isMajor site of drugs biotransformation is LIVERLIVER..
- 8. PRODRUGPRODRUG ► Chloral hydrateChloral hydrate ► PhenacetinPhenacetin ► 6- Mercaptopurine6- Mercaptopurine ► CortisoneCortisone ► PrednisonePrednisone ► Tal – AmpicillinTal – Ampicillin Piv – AmpicillinPiv – Ampicillin Bac – AmpicillinBac – Ampicillin ► ChlorazepateChlorazepate ► EnalaprilEnalapril ACTIVE DRUGACTIVE DRUG TrichloroethanolTrichloroethanol ParacetamolParacetamol 6- Mercaptopurine6- Mercaptopurine ribonucleotideribonucleotide HydrocortisoneHydrocortisone PrednisolonePrednisolone AmpicillinAmpicillin AmpicillinAmpicillin AmpicillinAmpicillin DesmethyldiazepamDesmethyldiazepam (Nordazepam)(Nordazepam) EnalaprilatEnalaprilat
- 9. ADVANTAGES OF ADMINISTRATION OF AADVANTAGES OF ADMINISTRATION OF A PRODRUGPRODRUG ► To make a drug tasteless and more stable e.g.To make a drug tasteless and more stable e.g. Propoxyphene hydrocloride (bitter and unstable)Propoxyphene hydrocloride (bitter and unstable) Propoxyphene Naphsylate (tasteless & stable).Propoxyphene Naphsylate (tasteless & stable). ► To make the drug more palatable e.g. ChloramphenicolTo make the drug more palatable e.g. Chloramphenicol Palmitate is given instead of Chloramphenicol.Palmitate is given instead of Chloramphenicol. ► To improve the rate of absorption of a drug e.g.To improve the rate of absorption of a drug e.g. Tal – Ampicillin, Piv – Ampicillin & Bac – Ampicillin areTal – Ampicillin, Piv – Ampicillin & Bac – Ampicillin are given instead of Ampicillin.given instead of Ampicillin.
- 10. ► To reduce the toxicity of a drug e.g. Tal – Ampicillin,To reduce the toxicity of a drug e.g. Tal – Ampicillin, Piv – Ampicillin & Bac – Ampicillin are given insteadPiv – Ampicillin & Bac – Ampicillin are given instead of Ampicillin.of Ampicillin. ► To increase the concentration of a drug at its site ofTo increase the concentration of a drug at its site of action e.g. Levodopa in place of Dopamine.action e.g. Levodopa in place of Dopamine. ► To increase the duration of action of a drug e.g.To increase the duration of action of a drug e.g. PhenothiazinePhenothiazine Fluphenazine (ester derivatives likeFluphenazine (ester derivatives like Fluphenazine enanthate, Fluphenazine decanoate).Fluphenazine enanthate, Fluphenazine decanoate).
- 11. BIOTRANSFORMATIONBIOTRANSFORMATION SITESSITES Liver, GIT, Lungs, Skin, Kidneys, Adrenals, Blood.Liver, GIT, Lungs, Skin, Kidneys, Adrenals, Blood. LiverLiver :-:- Meperidine, Pentazocine, Morphine, Nitroglycerine,Meperidine, Pentazocine, Morphine, Nitroglycerine, Lignocaine, Propranolol, Paracetamol, PrazosinLignocaine, Propranolol, Paracetamol, Prazosin GIT:-GIT:- Insulin. Catecholamines. Clonazepam, Chlorpromazine,Insulin. Catecholamines. Clonazepam, Chlorpromazine, Tyramine & SalbutamolTyramine & Salbutamol Lungs:-Lungs:- Prostanoids (prostaglandins & thromboxanes)Prostanoids (prostaglandins & thromboxanes) Plasma:-Plasma:- Suxamethonium (succinylcholine)Suxamethonium (succinylcholine) Procaine, Propanidid, Aspirin, ClofibrateProcaine, Propanidid, Aspirin, Clofibrate
- 12. FIRST-PASS EFFECTFIRST-PASS EFFECT OROR PRESYSTEMICPRESYSTEMIC METABOLISMMETABOLISM
- 13. Drugs undergoing extensive first-pass effectDrugs undergoing extensive first-pass effect ►LignocaineLignocaine ►NitroglycerineNitroglycerine ► InsulinInsulin ►Sympathomimetic catecholaminesSympathomimetic catecholamines ►MorphineMorphine ►MeperidineMeperidine ►PentazocinePentazocine ►ChlorpromazineChlorpromazine ►ClonazepamClonazepam
- 14. BIOTRANSFORMATIONBIOTRANSFORMATION REACTIONSREACTIONS The kidney cannot efficiently eliminateThe kidney cannot efficiently eliminate LipophilicLipophilic DrugsDrugs that readily cross cellthat readily cross cell membrane and are reabsorbed in the distalmembrane and are reabsorbed in the distal tubules.tubules. The lipid soluble agents must, therefore,The lipid soluble agents must, therefore, undergo biotransformation in the liver.undergo biotransformation in the liver.
- 15. BIOTRANSFORMATIONBIOTRANSFORMATION Drug biotransformation reactions are classified as:Drug biotransformation reactions are classified as: PHASE 1 REACTIONSPHASE 1 REACTIONS OxidationOxidation ReductionReduction HydrolysisHydrolysis
- 16. BIOTRANSFORMATIONBIOTRANSFORMATION PAHSE 2 REACTIONSPAHSE 2 REACTIONS Conjugation ReactionsConjugation Reactions GlucuronidationGlucuronidation SulfationSulfation AcetylationAcetylation Conjugates are generally Inactive and areConjugates are generally Inactive and are excreted rapidly in urine and fecesexcreted rapidly in urine and feces
- 20. TYPESTYPES a.a. Non-enzymaticNon-enzymatic b.b. EnzymaticEnzymatic b. Enzymaticb. Enzymatic a.a. MicrosomalMicrosomal b.b. Non-microsomalNon-microsomal BIOTRANSFORMATION
- 21. NON-ENZYMATICNON-ENZYMATIC BIOTRANSFORMATIONBIOTRANSFORMATION (HOFMANN ELIMINATION)(HOFMANN ELIMINATION) Mustine HClMustine HCl (Mechlorthamine) Ethyleneimonium(Mechlorthamine) Ethyleneimonium AtracuriumAtracurium Laudanosine & Quaternary acidLaudanosine & Quaternary acid HexamineHexamine (at acidic pH of urine) Formaldehyde(at acidic pH of urine) Formaldehyde ChlorazepateChlorazepate DesmethyldiazepamDesmethyldiazepam (at acidic pH in the stomach)(at acidic pH in the stomach)
- 22. PHASE - I REACTIONSPHASE - I REACTIONS (non-synthetic / catabolic)(non-synthetic / catabolic) I.I.OXIDATIONOXIDATION:-:- Microsomal Catalysed by Microsomal EnzymesMicrosomal Catalysed by Microsomal Enzymes Non-Microsomal Catalysed by Non-Microsomal EnzymesNon-Microsomal Catalysed by Non-Microsomal Enzymes A.A.MICROSOMAL OXIDATIONMICROSOMAL OXIDATION HYDROXYLATIONHYDROXYLATION a.a. HYDROXYLATION OF AROMATIC RING.HYDROXYLATION OF AROMATIC RING. PHENOBARBITONE P-HYDROXYPHENOBARBITONEPHENOBARBITONE P-HYDROXYPHENOBARBITONE b.b. ALIPHATIC HYDROXYLATIONALIPHATIC HYDROXYLATION MEPROBAMATE HYDRXYMEPROBAMATEMEPROBAMATE HYDRXYMEPROBAMATE N- DEALKYLATIONN- DEALKYLATION MEPHOBARBITONE PHENOBARBITONEMEPHOBARBITONE PHENOBARBITONE
- 23. O- DEALKYLATIONO- DEALKYLATION CODEINE MORPHINECODEINE MORPHINE N-OXIDATIONN-OXIDATION ANILINE NITROSOBENZENEANILINE NITROSOBENZENE SULFOXIDATIONSULFOXIDATION CHLORPROMAZINE CHLORPROMAZINE SULFOXIDECHLORPROMAZINE CHLORPROMAZINE SULFOXIDE DEAMINATIONDEAMINATION AMPHETAMINE PHENYLACETONEAMPHETAMINE PHENYLACETONE DESULFURATIONDESULFURATION PARATHION PARAOXONPARATHION PARAOXON
- 24. B.B. NON -NON - MICROSOMALMICROSOMAL OXIDATIONOXIDATION ETHANOLETHANOL ACETALDEHYDE ACETIC ACIDACETALDEHYDE ACETIC ACID HYPOXANTHINEHYPOXANTHINE XANTHINE URIC ACIDXANTHINE URIC ACID II.II. REDUCTIONREDUCTION CHLORAMPHENICOLCHLORAMPHENICOL ARYLAMINEARYLAMINE CHLORAL HYDRATECHLORAL HYDRATE TRICHLOROETHANOLTRICHLOROETHANOL OO III.III. HYDROLYSISHYDROLYSIS ((Drugs having ester linkageDrugs having ester linkage:-C—O—C):-C—O—C) PROCAINEPROCAINE PABA+DIETHYLAMINOETHANOLPABA+DIETHYLAMINOETHANOL ACETYLCHOLINEACETYLCHOLINE SUXAMETHONIUMSUXAMETHONIUM PropanididPropanidid
- 33. GDP
- 34. Hepatic microsomal enzymes (oxidation, conjugation) Extrahepatic microsomal enzymesExtrahepatic microsomal enzymes (oxidation, conjugation)(oxidation, conjugation) Hepatic non-microsomal enzymesHepatic non-microsomal enzymes (acetylation, sulfation, GSH,(acetylation, sulfation, GSH, alcohol/aldehyde dehydrogenase,alcohol/aldehyde dehydrogenase, hydrolysis, ox/red)hydrolysis, ox/red) Drug Metabolism
- 35. Microsomal Mixed Function Oxidase System showing Cytochrome P-450 cycle in drug oxidations COMPONENTS Cytochrome P-450 (P-450-Fe3+ ) Cytochrome P-450 Reductase (P-450-Fe2+ ) NADPH Flavoprotein Molecular Oxygen Membrane Lipid
- 36. Microsomal Mixed Function Oxidase System showing Cytochrome P-450 cycle in drug oxidations COMPONENTS Cytochrome P-450 (P-450-Fe3+ ) Cytochrome P-450 Reductase (P-450-Fe2+ ) NADPH Flavoprotein Molecular Oxygen Membrane Lipid Drug Substrate Figure 4-3. Cytochrome P-450 Cycle in drug oxidations (RH=par- ent drug ROH = oxidized metabolite Fp = flavoprotein, e = electron)
- 37. PHASE - II REACTIONS:PHASE - II REACTIONS: (CONJUGATION / SYNTHETIC / ANABOLIC REACTIONS)(CONJUGATION / SYNTHETIC / ANABOLIC REACTIONS) GLUCURONIDE CONJUGATION occurring with phenols, alcohols,GLUCURONIDE CONJUGATION occurring with phenols, alcohols, carboxylic acids and compounds containing amino or sulphydryl groupscarboxylic acids and compounds containing amino or sulphydryl groups Acetaminophen, Morphine, Digitoxin,Acetaminophen, Morphine, Digitoxin, GLYCINE CONJUGATIONGLYCINE CONJUGATION Salicylic acid, Aspirin, Benzoic AcidSalicylic acid, Aspirin, Benzoic Acid SULPHATE CONJUGATION occurring withSULPHATE CONJUGATION occurring with phenols, alcohols, and aromatic aminesphenols, alcohols, and aromatic amines Methyldopa, ParacetamolMethyldopa, Paracetamol ACETYLATIONACETYLATION Sulfonamides, INH, DapsoneSulfonamides, INH, Dapsone GLUTATHION CONJUGATIONGLUTATHION CONJUGATION Ethacrynic Acid, N-acetyl-p-benzoquinoneimine– reactive phase 1Ethacrynic Acid, N-acetyl-p-benzoquinoneimine– reactive phase 1 metabolite of acetaminophenmetabolite of acetaminophen METHYLATIONMETHYLATION Histamine , Adrenaline ,Nor-adrenaline, DopamineHistamine , Adrenaline ,Nor-adrenaline, Dopamine
- 38. PHASE -II REACTIONSPHASE -II REACTIONS Type ofType of ConjugationConjugation EndogenousEndogenous ReactantReactant TransferaseTransferase (Location)(Location) ExamplesExamples GlucuronidationGlucuronidation UDP-glucuronic acidUDP-glucuronic acid UDP-glucuronylUDP-glucuronyl transferasetransferase (microsomes)(microsomes) Morphine,Morphine, acetaminohpenacetaminohpen AcetylationAcetylation Acetyl – CoAAcetyl – CoA N-Acetyl transferaseN-Acetyl transferase (cytosol)(cytosol) Sulfonamides,Sulfonamides, isoniazidisoniazid GlutathioneGlutathione conjugationconjugation GlutathioneGlutathione GSH-S-transferaseGSH-S-transferase (cytosol, microsomes(cytosol, microsomes Ethacrynic acid,Ethacrynic acid, paracetamolparacetamol GlycineGlycine conjugationconjugation GlycineGlycine Acyl-CoA glycineAcyl-CoA glycine transferasetransferase (mitochondria)(mitochondria) Salicylic acid,Salicylic acid, benzonic acidbenzonic acid SulfateSulfate conjugationconjugation Phosphoadenosyl-Phosphoadenosyl- PhosphosulfatePhosphosulfate SulfotransferaseSulfotransferase (cytosol)(cytosol) Acetaminophen,Acetaminophen, methyldopamethyldopa MethylationMethylation S-S- AdenosylmethionineAdenosylmethionine TransmethylaseTransmethylase (cytosol)(cytosol) Dopamine,Dopamine, epinephrineepinephrine
- 39. DRUG BIOTRANSFORMATIONDRUG BIOTRANSFORMATION ► Pattern of ReactionsPattern of Reactions Phase-I Reaction is often followed by Phase-II Reaction.Phase-I Reaction is often followed by Phase-II Reaction. Drug or its metabolite produced from Phase-I Reaction is conjugated with an endogenousDrug or its metabolite produced from Phase-I Reaction is conjugated with an endogenous substrate during Phase-II Reaction.substrate during Phase-II Reaction. Phase-I Reaction (Drug or its Metabolite) Phase-II ReactionPhase-I Reaction (Drug or its Metabolite) Phase-II Reaction (Conjugation)(Conjugation)
- 40. ExceptionsExceptions ► In some cases Phase-II reaction may precede phase-I reaction. e.g.In some cases Phase-II reaction may precede phase-I reaction. e.g. IsoniazidIsoniazid Phase-II reaction (acetylation) take place first and is then followed byPhase-II reaction (acetylation) take place first and is then followed by phase-I reaction (Hydrolysis) as under:-phase-I reaction (Hydrolysis) as under:- Phase-II Phase-IPhase-II Phase-I Acetylation HydrolysisAcetylation Hydrolysis DRUG BIOTRANSFORMATIONDRUG BIOTRANSFORMATION INHINH N-acetyl INHN-acetyl INH Isonicotinic acid + acetylhydazineIsonicotinic acid + acetylhydazine
- 42. EFFECTS OF PHASE-I REACTIONS ON THEEFFECTS OF PHASE-I REACTIONS ON THE SOLUBILITY AND ACTIVITY OF A DRUGSOLUBILITY AND ACTIVITY OF A DRUG a.a. Lipid solubility of drugs partially converted into water solubility.Lipid solubility of drugs partially converted into water solubility. b.b. Sometimes water solubility may be decreasedSometimes water solubility may be decreased (diazepam desmethyldiazepam)(diazepam desmethyldiazepam) c.c. A drug may be inactivated, activity may be increased or activity may beA drug may be inactivated, activity may be increased or activity may be totally modified producing a highly toxic/ reactive / carcinogenictotally modified producing a highly toxic/ reactive / carcinogenic product.product. ► Parathion is converted into highly toxic paraoxon.Parathion is converted into highly toxic paraoxon. ► Acetaminophen (Paracetamol) is converted intoAcetaminophen (Paracetamol) is converted into highly toxic product (N-acetyl-p-highly toxic product (N-acetyl-p- benzoquinoneimine).benzoquinoneimine). ► Diazepam converted to Oxazepam witch is moreDiazepam converted to Oxazepam witch is more active.active. d. On biotransformation, a drug may yield more than one active/ inactive metabolites, some of them having longer half-life than that of the parent drug. Diazepam NordazepamDiazepam Nordazepam
- 44. Effects of Phase – II Reactions on DrugsEffects of Phase – II Reactions on Drugs aa. Generally. Generally Lipid solubility of drugs isLipid solubility of drugs is totally converted intototally converted into water solubilitywater solubility.. b.b. Drugs areDrugs are generally inactivatedgenerally inactivated.. c.c. Sometimes drug is activated e.g.Sometimes drug is activated e.g. Minoxidil is converted into active Minoxidil -o- sulphate.Minoxidil is converted into active Minoxidil -o- sulphate. Morphine converted into active Morphine-6-glucuronide.Morphine converted into active Morphine-6-glucuronide. dd. Conjugates may be secreted into the bile by an active transport. Conjugates may be secreted into the bile by an active transport process;process; may be split up by the intestinal bacteriamay be split up by the intestinal bacteria causingcausing release and reabsorption of active drug, thus establishingrelease and reabsorption of active drug, thus establishing entero-hepatic circulation of the active drug prolonging itsentero-hepatic circulation of the active drug prolonging its duration of actionduration of action e.g. Doxycyline, oral contraceptive combined-pill.e.g. Doxycyline, oral contraceptive combined-pill.
- 45. FACTORS MODIFYINGFACTORS MODIFYING BIOTRANSFORMATIONBIOTRANSFORMATION ►AGE.AGE. Chloramphenicol causes “Chloramphenicol causes “Gray Baby SyndromeGray Baby Syndrome”” Diazepam produces ”Diazepam produces ”Floppy Baby syndromeFloppy Baby syndrome”” ► ROUTE OF ADMINISTRATION.ROUTE OF ADMINISTRATION. 11stst Pass Effect:-Pass Effect:- ► LignocaineLignocaine ► NitroglycerineNitroglycerine ► InsulinInsulin ► Sympathomimetic catecholaminesSympathomimetic catecholamines ► MorphineMorphine ► MeperidineMeperidine ► PentazocinePentazocine ► ChlorpromazineChlorpromazine ► ClonazepamClonazepam
- 46. GENETIC FACTORS.GENETIC FACTORS. Atypical pseudocholinesterase:-Atypical pseudocholinesterase:- SuccinylcholineSuccinylcholine (Suxamethonium) is hydrolysed by pseudocholinesterase.(Suxamethonium) is hydrolysed by pseudocholinesterase. Genetically abnormal pseudocholinesterase Apnoea.Genetically abnormal pseudocholinesterase Apnoea. Acetylation status:Acetylation status: Slow & Fast acetylators Inherited as anSlow & Fast acetylators Inherited as an autosomal recessive trait Slow acetylator phenotype – about 50autosomal recessive trait Slow acetylator phenotype – about 50 % 0f blacks & white in the USA Much less common in Asians &% 0f blacks & white in the USA Much less common in Asians & eskimoseskimos Genetically determined defects in the CYP dependent oxidativeGenetically determined defects in the CYP dependent oxidative metabolism of:metabolism of: Debrisoquin, Phenacetin, Phenformin (extensive metabolizersDebrisoquin, Phenacetin, Phenformin (extensive metabolizers and poor metabolizers)and poor metabolizers) Hydroxylation of anticonvulsant Mephenytoin:Hydroxylation of anticonvulsant Mephenytoin: PoorPoor hydroxylators & Fast hydroxylatorshydroxylators & Fast hydroxylators
- 47. ► PATHOLOGICAL STATES OF:PATHOLOGICAL STATES OF: ► LIVER:-LIVER:- acute & chronic liver diseases, CA of liver depressacute & chronic liver diseases, CA of liver depress metabolism of chloramphnical & diazepam increasing their plasmametabolism of chloramphnical & diazepam increasing their plasma half-life.half-life. ► CVS:-CVS:- CCF decreases hepatic perfusion decreasing the clearance ofCCF decreases hepatic perfusion decreasing the clearance of lidocaine.lidocaine. ► LUNGS:-LUNGS:- In pulmonary diseases, metabolism of procainamide andIn pulmonary diseases, metabolism of procainamide and aminopyirne is depressed.aminopyirne is depressed. ► ENDOCRINES:-ENDOCRINES:- Hypothyroidism decreases the metabolism ofHypothyroidism decreases the metabolism of digoxin, methimazole, practolol increasing their plasma half-life,digoxin, methimazole, practolol increasing their plasma half-life, reverse happens in hyperthyroridism.reverse happens in hyperthyroridism. ► NUTRITIONAL STATUS:NUTRITIONAL STATUS: ► STARVATIONSTARVATION ► MALNUTRITIONMALNUTRITION ► SPECIESSPECIES (Phenylbutazone, Pethidine, Barbiturates)(Phenylbutazone, Pethidine, Barbiturates) ► SEXSEX (Hexobarbitone, BDZs, Oestrogens,(Hexobarbitone, BDZs, Oestrogens, Salicylates)Salicylates)
- 48. DRUG INTERACTIONS:DRUG INTERACTIONS: Enzyme inductionEnzyme induction Enzyme inhibitionEnzyme inhibition Competitive substrate inhibitionCompetitive substrate inhibition
- 49. Enzyme InductionEnzyme Induction Enzyme InducersEnzyme Inducers PHENOBARBITONEPHENOBARBITONE PHENYLBUTAZONEPHENYLBUTAZONE PHENYTOINPHENYTOIN MEPROBAMATEMEPROBAMATE RIFAMPICINRIFAMPICIN CARBAMAZEPINECARBAMAZEPINE Ch. alcoholismCh. alcoholism BENZOBENZO αα pyrene in Tobacco smokepyrene in Tobacco smoke ClofibrateClofibrate DEXAMETHASONEDEXAMETHASONE Chronic AlcoholismChronic Alcoholism WarfarinWarfarin
- 50. ENZYME INHIBITORSENZYME INHIBITORS TypesTypes • Competitive inhibitorsCompetitive inhibitors quinidinequinidine • Reversible non - Competitive inhibitorsReversible non - Competitive inhibitors –– KETOCONAZOLEKETOCONAZOLE • Suicide inhibitors- GestodeneSuicide inhibitors- Gestodene CIMETIDINECIMETIDINE KETOCONAZOLEKETOCONAZOLE ERYTHOMYCINERYTHOMYCIN CHLORAMPHENICOLCHLORAMPHENICOL ISONIAZIDISONIAZID PROPYLTHIOURACILPROPYLTHIOURACIL ALLOBARBITONEALLOBARBITONE Suicide inhibitorsSuicide inhibitors SECOBARBITONESECOBARBITONE SPIRONOLACTONESPIRONOLACTONE Inhibitors of intestinal P-gycoprotein (P-gp)Inhibitors of intestinal P-gycoprotein (P-gp) VerapamilVerapamil Certain components of grape fruit juiceCertain components of grape fruit juice Drugs expelled by P-gp are digoxin, cyclosporineDrugs expelled by P-gp are digoxin, cyclosporine
- 51. Competitive substrate inhibitionCompetitive substrate inhibition ►Sulphonamides inhibit the metabolism ofSulphonamides inhibit the metabolism of PhenytoinPhenytoin ►Phenylbutazone & coumarins inhibit thePhenylbutazone & coumarins inhibit the metabolism of tolbutamide.metabolism of tolbutamide.
- 52. ►CIRCADIAN RHYTHMCIRCADIAN RHYTHM ►ENVIRONMENTAL FACTORS: Smoking,ENVIRONMENTAL FACTORS: Smoking, Alcoholism, Exposure to Pesticides.Alcoholism, Exposure to Pesticides. ►CLIMATE & ALTITUDECLIMATE & ALTITUDE
- 53. BIOTRANSFORMATIONBIOTRANSFORMATION BIOTRANSFORMATIONBIOTRANSFORMATION usually results inusually results in the loss of Pharmacological activity, but therethe loss of Pharmacological activity, but there are exceptions.are exceptions. PRODRUGPRODRUG →→ ACTIVE DRUGACTIVE DRUG DRUGDRUG →→ MORE ACTIVE COMPOUNDMORE ACTIVE COMPOUND DRUGDRUG →→ TOXIC COMPOUNDTOXIC COMPOUND
