Unit 1 lesson 11ppt
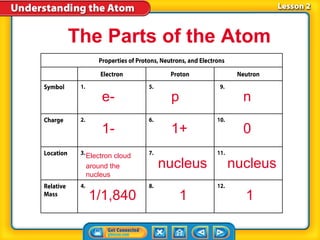
- 1. The Parts of the Atom e- p n 1- 1+ 0 Electron cloud around the nucleus nucleus nucleus 1/1,840 1 1
- 2. Which two particles are responsible for the mass of an atom? Protons and Neutrons Which two particles are responsible for the charge of an atom? Protons and Electrons
- 4. 26 Atomic Number Atomic Number Fe Iron 55.845 Atomic Mass Atomic Mass Atomic Number = 26 26 Atomic Mass = 55.845 55.845 Mass # = 56 56 # of Protons = 26 26 # of Neutrons = 30 30 # of Electrons = 26 26
- 5. Notes and Guided Practice
- 6. Different Elements—Different Numbers of Protons the number of protons The type of the number of electrons element
- 7. What are the atomic numbers of carbon, nitrogen and oxygen? Carbon = 6, Nitrogen = 7, Oxygen = 8 Explain the difference between an oxygen atom and a carbon atom. Oxygen has 8 protons. Carbon has 6 protons.
- 8. Neutrons and Isotopes • Isotopes are atoms of the same element that have different numbers of neutrons. Most elements have several isotopes. Same Different
- 9. How do the carbon isotopes differ? Carbon isotopes have different numbers of neutrons
- 10. Atomic Number vs. Mass Number The number of The number of protons protons and neutrons Hint : # of neutrons = mass # - protons
- 11. Average Atomic Mass The average atomic mass of an element is the average mass of the element’s isotopes, weighted according to the abundance of each isotope.
- 12. How is the average atomic mass calculated? The abundance of each isotope is changed to decimal form. The mass of each isotope is multiplied by it’s corresponding decimal percentage. All the values are added together to determine the average atomic mass 0.989 x 12 = 11.868 0.0101 x 13= + 0.143 12.01