Spectroscopy
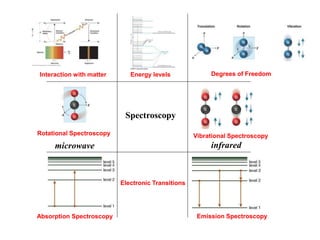
- 1. Degrees of Freedom Rotational Spectroscopy Electronic Transitions Vibrational Spectroscopy microwave infrared Interaction with matter Energy levels Spectroscopy Absorption Spectroscopy Emission Spectroscopy
- 2. Introduction to Matters Rotational Spectroscopy Vibrational Spectroscopy Absorption and emission Spectroscopy Lakowicz, “Principles of Fluorescence Spectroscopy”, Springer Publishers, 3rd Edition, 2011 Atkins, Physical Chemistry, 9th edition, 2009 Banwell & McCash, Fundamentals of Molecular Spectroscopy, 4th edition, 1996 Moog, Spencer and farrell, Physical Chemistry: A Guided Inquiry: Atoms, Molecules, and Spectroscopy, 2003
- 3. • The sun produces a full spectrum of electromagnetic radiation http://csep10.phys.utk.edu/astr162/lect/light/spectrum.html http://kr.blog.yahoo.com/bmw26z/2188
- 4. Components of Electro Magnetic Radiation v
- 5. Two Components of EM Radiation • Electrical field (E): varies in magnitude in a direction perpendicular to the direction of propagation • Magnetic field (M): at right angle to the electrical field, is propagated in phase with the electrical field • Wavelength (l), distance from one wave crest to another • Frequency (n), No. of crests passing a fixed point/ given time • Amplitude, height of each peak (watts/sq. meter • The speed of EM energy “c” 300,000km/second, c = nl where l and n are inversely related
- 6. Interaction of radiation with matter • If there are no available quantized energy levels matching the quantum energy of the incident radiation, then the material will be transparent to that radiation Wavelength
- 7. Fate of molecule? • Non-radiative transition: M* + M M + M + heat • Spontaneous emission: M* M + hn (very fast for large DE) • Stimulated emission (opposite to stimulated absorption) These factors contribute to linewidth & to lifetime of excited state.
- 8. Molecular Motion and Spectroscopy Study of Interaction of Matter and Light (Photon) • Molecular Spectroscopy Information about molecules such as geometry and energy levels are obtained by the interaction of molecules and photons • Molecular motions: Translation, Rotation, Vibration determines the energy levels for the absorption or emission of photons
- 9. Electronic, Vibrational, and Rotational Energy Levels of a Diatomic Molecule Exercise: Indicate the molecular state in which it is electronically in the ground state, vibrationally in the first excited state, and rotationally in the ground state.
- 11. Microwave interactions • Quantum energy of microwave photons (0.00001-0.001 eV) matches the ranges of energies separating quantum states of molecular rotations and torsion • Note that rotational motion of molecules is quantized, like electronic and vibrational transitions associated absorption/emission lines • Absorption of microwave radiation causes heating due to increased molecular rotational activity
- 13. Types of Rigid Rotors • A schematic illustration of the classification of rigid rotors.
- 14. A diatomic molecule can rotate around a vertical axis. The rotational energy is quantized. RIGID ROTOR Figure 40-16 goes here.
- 15. THE RIGID ROTOR A diatomic molecule may be thought of as two atoms held together with a massless, rigid rod (rigid rotator model). o r1 | r2 m1 m2 o • Consider a diatomic molecule with different atoms of mass m1 and m2, whose distance from the center of mass are r1 and r2 respectively • The moment of inertia of the system about the center of mass is: I m1r1 2 m2r2 2
- 16. The Definition of Moment of Inertia • In this molecule – three identical atoms attached to the B atom – three different but mutually identical atoms attached to the C atom. • Centre of mass lies on the C3 axis • Perpendicular distances are measured from the axis passing through the B and C atoms.
- 17. Rotational levels )1( 2 22 2 2 22 JJ h L momentumangularLwhere I LI Er • The classical expression for energy of rotation is :In the harmonic oscillator model, the energy was all potential energy. In the rigid rotor, it’s all kinetic energy: • • where J is the rotational quantum number constantrotationalthe 8 )1()1( 22 1 )( 2 2 Ic h B JJBchJJ h I JE n n
- 18. Quantization of Rotational Energy 2 ( ) 8 h B cI V = 0 cyclic boundary condition: Ψ(2π + θ) = Ψ(θ) By solving Schrodinger equation for rotational motion the rotational energy levels are Rotational energy levels in wavenumber (cm-1)
- 19. Spacing between adjacent rotational levels j and j-1)
- 20. The Gross Selection Rule for Rotations • A rotating polar molecule looks like an oscillating dipole which can stir the electromagnetic field into oscillation. • Classical origin of the gross selection rule for rotational transitions.
- 21. /h hc n lD 1 1 ( ) 1 ( ) j j absorption j emission D D D Rotational Spectroscopy (1) Bohr postulate (2) Selection Rule
- 22. Vibrational Motion: Molecular Calisthenics Harmonic oscillator ( )eF k r r 141 2 10vib s n A molecule vibrates ~50 times during a molecular day (one rotation)
- 23. Quantization of Vibrational Energy By solving Schrodinger equation for vibrational motion, Vibrational energy level Zero point energy Spacing between adjacent vibrational sates where is a vibrational quantum number
- 26. ROTATIONAL SPECTRUM Selection Rule: Apart from Specific rule- DJ 1, Gross rule- the molecule should have a permanent electric dipole moment, m . Thus, homonuclear diatomic molecules do not have a pure rotational spectrum. Heteronuclear diatomic molecules do have rotational spectra 1 1 ( ) 1 ( ) j j absorption j emission D D D
- 27. Appearance of rotational spectrum We can calculate the energy corresponding to rotational transitions D E=EJ’ –EJ for Or generally: J J + 1 = B(J+1)(J+2) - BJ(J+1) = 2B(J+1) cm-1 Microwave absorption lines should appear at J = 0 J = 1 : = 2B - 0 = 2B cm-1 J = 1 J = 2 : = 4B cm-1 Note that the selection rule is DJ = 1, where + applies to absorption and - to emission. ν 1D initialfinal JJJ ν
- 28. Relative Intensities of rotation spectral lines Now we understand the locations (positions) of lines in the microwave spectrum, we can see which lines are strongest. J BJ(J+1) J=0 0 Intensity depends upon two factors:
- 29. Intensity depends upon two factors: 1.Greater initial state population gives stronger spectral lines.This population depends upon temperature, T. k = Boltzmann’s constant, 1.380658 x 10-23 J K-1 (k = R/N) We conclude that the population is smaller for higher J states. kT νhc exp kT E exp N N J 0 J cmK1.52034 k hc T ν1.52034 e N N o J
- 30. 2. Intensity also depends on degeneracy of initial state. (degeneracy = existence of 2 or more energy states having exactly the same energy) Each level J is (2J+1) degenerate population is greater for higher J states. To summarize: Total relative population at energy EJ (2J+1) exp (-EJ / kT) & maximum population occurs at nearest integral J value to : Look at the values of NJ/N0 in the figure, .
- 31. Plot of population of rotational energy levels versus value of J. B = 10cm-1 max. pop. J0 Pop(2J+1)e(-BJ(J+1)hc/kT)
- 32. MCWE or Rotational Spectroscopy Classification of molecules • Based on moments of inertia, I=mr2 – IA IB IC very complex eg H2O – IA = IB = IC no MCWE spectrum eg CH4 – IA IB = IC complicated eg NH3 – IA = 0, IB = IC linear molecules eg NaCl E J J I J M JJ J 1 2 012 0 1 2 with also, , , , ,
- 34. Vibrational Spectra Molecules are not Static Vibration of bonds occurs in the liquid, solid and gaseous phase Vibrating Energy Frequency (and the appropriate frequencies for molecular vibrations are in the Infrared region of the electromagnetic spectrum Vibrations form therefore, a fundamental basis for spectroscopy in chemistry--the bonds are what makes the chemistry work in structure and function For Organic Chemistry the most important uses of these vibrations is for analysis of: •functional groups •structural identity, “fingerprinting”
- 35. What Kind of vibrations are These? Bonds can……. Stretch Bend Wag (rock) These can number into the hundreds. Some are symmetrical, some antisymmetrical and many are coupled across the molecule Can be calculated. One easy approximation is: m n k12 103.5 - ´= 21 21 mm mm + =m The “reduced mass” where m1, m2 are the masses on either side of vibration k is the “force constant”, like the Hookes Law restoring force for a spring. Known and tabulated for different vibrations
- 36. The Fundamentals R R H H R R H H R R H H R R H H R R H H R R H H antisymmetric symmetric rockingscissoring in-plane bending stretching out-of-plane bending wagging twisting These oscillating electric dipoles match in frequency the incoming e-field oscillations of IR light. All the simple possibilities. For n atoms in a molecule; – Linear: 3n – 5 modes – Non-linear: 3n – 6 modes – Example for a methylene,given n=3 While useful, this oversimplifies, since molecular orbital picture requires that atoms can’t vibrate without affecting the rest of the molecule.
- 37. A Functional Group Chart O-H str NH str COO-H =C-H str Csp3-H C-H -(C=O)-H CN CC C=O -C=N -C=C phenyl C-O C-N F C-X 4000 3600 3200 2800 2400 2000 1600 1200 800 group Br Cl
- 38. Regions of Frequencies Near -to visible- IR (NIR) Combination bands 3.8 x 1014 to 1.2 x 1014 12800 to 4000 0.78 to 2.5 Mid Infrared Fundmental bands for organic molecules 1.2 x 1014 to 6.0 x 1012 4000 to 200 2.5 to 50 Far IR Inorganics organometallics 6.0 x 1012 to 3.0 x 1011 200 to 10 50 to 1000 Spectral Region Frequency(Hz) Wavenumber(cm-1) Wavelength (l,mm)
- 39. Looking at a Spectrum Divide the spectrum in to two regions: 4000 cm-1 1600 cm-1 most of the stretching bands, specific functional groups (specific atom pairs). This is the “functional group” region. 1600 cm-1 400 cm-1 Many band of mixed origin. Some prominent bands are reliable. This is the “fingerprint” region. Use for comparison with literature spectra. Wavenumber is cm-1=104/l(m)
- 40. What kinds of Bonds Absorb in which Regions? Bending is easier than stretching-- happens at lower energy (lower wavenumber) Bond Order is reflected in ordering-- triple>double>single (energy) with single bonds easier than double easier than triple Heavier atoms move slower than lighter ones The k in the frequency equation is in mDyne/Å of displacement Single bond str 3-6 mD/Å Double bond str. 10-12 mD/Å Triple Bond 15-18 mD/Å
- 41. Effects of conjugation Lowers to 1715 cm-1 Similar, to 1715 cm-1 Raises to 1770 cm-1 : Weakens DB character Strengthens DB character (inductive over resonance)
- 42. Degrees of Freedom: Translation, Rotation, and Vibration Consider a single Ar atom moving in 3-D space: - Moving motion is referred to as Translation - To analyze the translation of an Ar, we need to know position (x, y, z) and momentum (px, py, pz) Where it is Where it is headed - Each coordinate-momentum pair [for example, (x,px)] is referred to as a Degree of Freedom (DF) - An Ar atom moving through 3-D space has three DFs N argon atoms possesses 3N DFs: All translational DFs
- 45. Center of Mass (Balanced Point) - A point mass that can represent the molecule - Motion of the center of mass requires 3 DFs to describe it - In general, regardless of its size or complexity, a molecule has 3 translational DFs - Thus, (3N – 3) DFs for the internal motions of rotation and vibration
- 46. Rotational and vibrational DFs N atomic Linear Molecule N atomic Non-Linear Molecule Rotation 2 DFs 3 DFs Vibration 3N – 5 3N - 6
- 47. Vibrational Spectroscopy Vibrational selection rule 1 1 ( ) 1 ( ) absorption emission D D D
- 49. The Vibrations of CO2. • The stretching modes are not independent, and if one CO group is excited the other begins to vibrate. • The symmetric and antisymmetric stretches are independent, and one can be excited without affecting the other: they are normal modes. • The two perpendicular bending motions are also normal modes.
- 50. The Normal Modes of Water • The three normal modes of H2O. The mode v2 is predominantly bending, and occurs at lower wavenumber than the other two.
- 51. Absorption and Emission Spectroscopy
- 52. Electronic Transitions in Molecules Molecular Orbital (MO) Theory for C2H4 molecule, UV or Visible spectral region
- 54. Department of Chemistry, KAIST Fate of Excited Electronic States
- 55. Reflection and Scattering Losses Introduction to Absorption
- 56. LAMBERT-BEER LAW ionconcentrat pathlength tyabsorptivi loglog 0 0 c b a kcabcA P P TA P P P P T solvent solution Power of radiation after passing through the solvent Power of radiation after passing through the sample solution
- 57. Beer’s law and mixtures • Each analyte present in the solution absorbs light! • The magnitude of the absorption depends on its • A total = A1+A2+…+An • A total = 1bc1+2bc2+…+nbcn • If 1 = 2 = n then simultaneous determination is impossible • Need nl’s where ’s are different to solve the mixture
- 58. Molecular Transitions for UV-Visible Absorptions • What electrons can we use for these transitions?
- 59. Spectral nomenclature of shifts
- 60. Introduction to Emission • Luminescence: emission of photons from electronically excited states of atoms, molecules, and ions. • Fluorescence: Average lifetime from <10—10 to 10—7 sec from singlet states. • Phosphorescence: Average lifetime from 10—5 to >10+3 sec from triplet excited states.
- 61. Importance of Emission Spectroscopy • Sensitivity to local electrical environment – polarity, hydrophobicity • Track (bio-)chemical reactions • Measure local friction (microviscosity) • Track solvation dynamics • Measure distances using molecular rulers: fluorescence resonance energy transfer (FRET)
- 62. Photophysics: Jablonski Diagram Kasha’s rule, Internal Conversion, Intersystem crossing, fluorescence, phosphorescence
- 63. Principles • Interaction of photons with molecules results in promotion of valence electrons from ground state orbitals to high energy levels. • The molecules are said to be in excited state. • Molecules in excited state do not remain there long but spontaneously relax to more stable ground state.
- 64. • The relaxation process is brought about by collisional energy transfer to solvent or other molecules in the solution. • Some excited molecules however return to the ground state by emitting the excess energy as light. • This process is called fluorescence.
- 65. Light Amplification by Stimulated Emission of Radiation
- 66. In a laser…. Three key elements in a laser •Pumping process prepares amplifying medium in suitable state •Optical power increases on each pass through amplifying medium •If gain exceeds loss, device will oscillate, generating a coherentoutput
