Poster_Maolin for 2013 RESEARCH WEEK
•Als PPT, PDF herunterladen•
0 gefällt mir•84 views
Melden
Teilen
Melden
Teilen
Empfohlen
Empfohlen
Weitere ähnliche Inhalte
Ähnlich wie Poster_Maolin for 2013 RESEARCH WEEK
Ähnlich wie Poster_Maolin for 2013 RESEARCH WEEK (12)
Lattice Energy LLC - Electroweak nuclear catalysis and chemical catalysis are...

Lattice Energy LLC - Electroweak nuclear catalysis and chemical catalysis are...
Electrodeposition of flower-like nickel oxide on single layer graphene

Electrodeposition of flower-like nickel oxide on single layer graphene
Conducting polymer based flexible super capacitors [autosaved]![Conducting polymer based flexible super capacitors [autosaved]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Conducting polymer based flexible super capacitors [autosaved]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Conducting polymer based flexible super capacitors [autosaved]
Increase In Electrical And Thermal Conductivities Of Doped Polymers Dependent...

Increase In Electrical And Thermal Conductivities Of Doped Polymers Dependent...
Charge Transfer Complexation and Excited State Interactions in Porphyrin-Ag N...

Charge Transfer Complexation and Excited State Interactions in Porphyrin-Ag N...
Poster_Maolin for 2013 RESEARCH WEEK
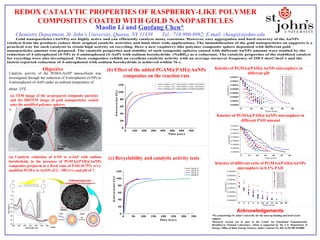
- 1. REDOX CATALYTIC PROPERTIES OF RASPBERRY-LIKE POLYMER COMPOSITES COATED WITH GOLD NANOPARTICLES Maolin Li and Guofang Chen* Chemistry Department, St. John’s University, Queens, NY 11439 Tel.: 718-990-8092; E-mail: cheng@stjohns.edu Objective Acknowledgements SEM Top View Enlarged View Kinetics of PGMA@PAH@AuNPs microsphere at different pH Kinetics of PGMA@PAH@AuNPs microsphere in different PAH amount Kinetics of different ratio of PGMA@PAH@AuNPs microsphere in 0.1% PAH •We acknowledge St. John's University for the start-up funding and Seed Grant support. •Research carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886 0 10 20 30 40 50 60 0.00000 0.00002 0.00004 0.00006 0.00008 0.00010 0.00012 0.00014 0.1PAH-0.2mL 0.1PAH-0.4mL 0.1PAH-0.6mL 0.5PAH-0.2mL 0.5PAH-0.4mL 0.5PAH-0.6mL 0.75PAH-0.2mL 0.75PAH-0.4mL 0.75PAH-0.6mL 1.0PAH-0.2mL 1.0PAH-0.4mL 1.0PAH-0.6mL C A (mol/L) Time (min) 0 10 20 30 40 50 0.00000 0.00002 0.00004 0.00006 0.00008 0.00010 0.00012 0.00014 pH3-0.2mL pH3-0.4mL pH3-0.6mL pH5-0.2mL pH5-0.4mL pH5-0.6mL pH7-0.2mL pH7-0.4mL pH7-0.6mL pH9-0.2mL pH9-0.4mL pH9-0.6mL pH11-0.2mL pH11-0.4mL pH11-0.6mL C A (mol/L) Time (min) 0 2 4 6 8 10 12 14 16 18 20 0.00000 0.00004 0.00008 0.00012 0.00016 0.00020 0.00024 0.00028 100/1-0.2mL 100/1-0.4mL 100/1-0.6mL 100/2-0.2mL 100/2-0.4mL 100/2-0.6mL 100/4-0.2mL 100/4-0.4mL 100/4-0.6mL 100/10-0.2mL 100/10-0.4mL 100/10-0.6mL C A (mol/L) Time (min) Catalytic activity of the PGMA-AuNP nanocolloids was investigated through the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AnP) under an ambient temperature of about 25℃. (a) TEM image of the as-prepared composite particles and (b) HRTEM image of gold nanoparticles coated onto the modified polymer spheres. (a) Catalytic reduction of 4-NP to 4-AnP with sodium borohydride in the presence of PGMA@PAH@AuNPs composites prepared at a fixed ratio of PAH (0.75% w/v)- modified PGMA to AuNPs of 2 : 100 (v/v) and pH of 7. 0 100 200 300 400 500 600 700 0 20 40 60 80 100 120 Conversion(%) Time (s ec) 6.5663 x 10 -12 mole AuNPs; 0.6067 mg PGMA 1.6416 x 10 -12 mole AuNPs; 0.1517 mg PGMA 8.2079 x 10 -13 mole AuNPs; 0.0758 mg PGMA (b) Effect of the added PGAM@PAH@AuNPs composites on the reaction rate 0 50 100 150 200 250 300 350 0 20 40 60 80 100 120 Conversion(%) Time (s ec) Cycle 1 Cycle 2 Cycle 3 Cycle 4 (c) Recyclability and catalytic activity tests