Melden
Teilen
Downloaden Sie, um offline zu lesen
Empfohlen
Empfohlen
Learn what is necessary for P&PC and how to integrate it seamlessly with the rest of your Quality Management System by attending this webinar.Production and Process Control: Building a Robust System for Medical Device C...

Production and Process Control: Building a Robust System for Medical Device C...OnlineCompliance Panel
Weitere ähnliche Inhalte
Was ist angesagt?
Learn what is necessary for P&PC and how to integrate it seamlessly with the rest of your Quality Management System by attending this webinar.Production and Process Control: Building a Robust System for Medical Device C...

Production and Process Control: Building a Robust System for Medical Device C...OnlineCompliance Panel
Was ist angesagt? (19)
Acceptance Activities under the FDA QSRs-Online Webinar

Acceptance Activities under the FDA QSRs-Online Webinar
SAP Consulting Services in Pharmaceuticals | Apprisia

SAP Consulting Services in Pharmaceuticals | Apprisia
Using RTLS to Overcome Business Challenges in Today's OR

Using RTLS to Overcome Business Challenges in Today's OR
Production and Process Control: Building a Robust System for Medical Device C...

Production and Process Control: Building a Robust System for Medical Device C...
Andere mochten auch
Andere mochten auch (12)
Ähnlich wie Infographic_APV_22July2015
Raaj-GPRAC is a Thane-Mumbai (India) based agency.
Our offerings includes but not limited to making Regulatory filing strategy, Preparation of pharmaceutical product registration dossier, filing assistance, response to regulatory queries, life cycle management, regulatory compliance audit and Training.
We offer flexible and need based services to meet customer/client requirements so as to save time and cost.
Raaj Global Pharma Regulatory Affairs Consultants Thane-mumbai profile-updat...

Raaj Global Pharma Regulatory Affairs Consultants Thane-mumbai profile-updat...Rajashri Survase Ojha
Ähnlich wie Infographic_APV_22July2015 (20)
Software as a Medical Device (SaMD) Challenges and Opportunities for 2021 and...

Software as a Medical Device (SaMD) Challenges and Opportunities for 2021 and...
Applying Technologies Across the End-to-End Pharmacovigilance Process to Incr...

Applying Technologies Across the End-to-End Pharmacovigilance Process to Incr...
The Benefits of a Seamless IRT and EDC Integration in Clinical Trial Execution

The Benefits of a Seamless IRT and EDC Integration in Clinical Trial Execution
Role of TGA Plenary session Sponsor Information and Training day

Role of TGA Plenary session Sponsor Information and Training day
Simplifying Postmarket Surveillance: Introducing Veeva Vault Product Surveill...

Simplifying Postmarket Surveillance: Introducing Veeva Vault Product Surveill...
Simplifying Postmarket Surveillance: Introducing Veeva Vault Product Surveill...

Simplifying Postmarket Surveillance: Introducing Veeva Vault Product Surveill...
Raaj Global Pharma Regulatory Affairs Consultants Thane-mumbai profile-updat...

Raaj Global Pharma Regulatory Affairs Consultants Thane-mumbai profile-updat...
Computer Software Assurance (CSA): Understanding the FDA’s New Draft Guidance

Computer Software Assurance (CSA): Understanding the FDA’s New Draft Guidance
Transforming Pharmacovigilance from Operational to Scientifically Driven

Transforming Pharmacovigilance from Operational to Scientifically Driven
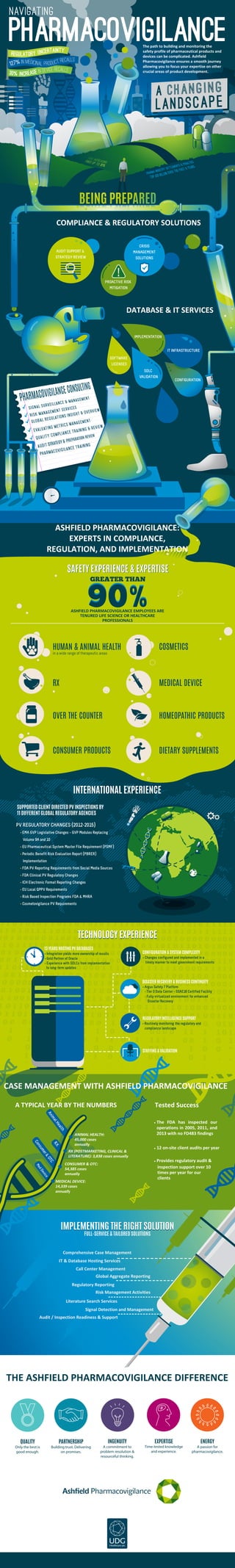
Infographic_APV_22July2015
- 1. CONFIGURATION Pharmacovigilance navigating The path to building and monitoring the safety profile of pharmaceutical products and devices can be complicated. Ashfield Pharmacovigilance ensures a smooth journey allowing you to focus your expertise on other crucial areas of product development. PV REGULATORY CHANGES (2012-2015) • EMA GVP Legislative Changes – GVP Modules Replacing Volume 9A and 10 • EU Pharmaceutical System Master File Requirement (PSMF) • Periodic Benefit Risk Evaluation Report (PBRER) Implementation • FDA PV Reporting Requirements from Social Media Sources • FDA Clinical PV Regulatory Changes • ICH Electronic Format Reporting Changes • EU Local QPPV Requirements • Risk Based Inspection Programs FDA & MHRA • Cosmetovigilance PV Requirements COMPLIANCE & REGULATORY SOLUTIONS DATABASE & IT SERVICES AUDIT SUPPORT & STRATEGY REVIEW PROACTIVE RISK MITIGATION CRISIS MANAGEMENT SOLUTIONS ! SOFTWARE LICENSES IT INFRASTRUCTURE ASHFIELD PHARMACOVIGILANCE EMPLOYEES ARE TENURED LIFE SCIENCE OR HEALTHCARE PROFESSIONALS GREATER THAN IMPLEMENTATION SDLC VALIDATION HUMAN & ANIMAL HEALTH in a wide range of therapeutic areas COSMETICS MEDICAL DEVICE HOMEOPATHIC PRODUCTS DIETARY SUPPLEMENTS RX OVER THE COUNTER CONSUMER PRODUCTS INTERNATIONAL EXPERIENCEINTERNATIONAL EXPERIENCE SUPPORTED CLIENT DIRECTED PV INSPECTIONS BY 11 DIFFERENT GLOBAL REGULATORY AGENCIES ASHFIELD PHARMACOVIGILANCE: EXPERTS IN COMPLIANCE, REGULATION, AND IMPLEMENTATION CONFIGURATION & SYSTEM COMPLEXITY • Changes configured and implemented in a timely manner to meet government requirements 13 YEARS HOSTING PV DATABASES • Integration yields more ownership of results • Gold Partner of Oracle • Experience with SDLCs from implementation to long-term updates DISASTER RECOVERY & BUSINESS CONTINUITY • Argus Safety 7 Platform - Tier 3 Data Center – SSAE16 Certified Facility - Fully virtualized environment for enhanced Disaster Recovery SAFETY EXPERIENCE & EXPERTISESAFETY EXPERIENCE & EXPERTISE REGULATORY INTELLIGENCE SUPPORT • Routinely monitoring the regulatory and compliance landscape STAFFING & VALIDATION IT & Database Hosting Services Call Center Management Risk Management Activities Literature Search Services Signal Detection and Management Audit / Inspection Readiness & Support Global Aggregate Reporting Regulatory Reporting Comprehensive Case Management QUALITY Only the best is good enough. PARTNERSHIP Building trust. Delivering on promises. INGENUITY A commitment to problem resolution & resourceful thinking. EXPERTISE Time-tested knowledge and experience. ENERGY A passion for pharmacovigilance. • The FDA has inspected our operations in 2005, 2011, and 2013 with no FD483 findings • 12 on-site client audits per year • Provides regulatory audit & inspection support over 10 times per year for our clients MEDICAL DEVICE: 14,339 cases annually CONSUMER & OTC: 54,385 cases annually RX (POSTMARKETING, CLINICAL & LITERATURE): 3,838 cases annually ANIMAL HEALTH: 45,000 cases annually RX AnimalHealth Consumer&OTC MedDevice CASE MANAGEMENT WITH ASHFIELD PHARMACOVIGILANCE TECHNOLOGY EXPERIENCETECHNOLOGY EXPERIENCE IMPLEMENTING THE RIGHT SOLUTIONIMPLEMENTING THE RIGHT SOLUTION FULL-SERVICE & TAILORED SOLUTIONS THE ASHFIELD PHARMACOVIGILANCE DIFFERENCE 30% INCREASE IN DEVICE RECALLS 127% IN MEDICINAL PRODUCT RECALLS REGULATORY UNCERTAINTY Tested SuccessA TYPICAL YEAR BY THE NUMBERS