As ocr biology revision pack unit f212 edited
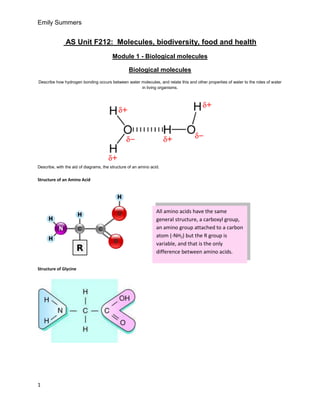
- 1. Emily Summers AS Unit F212: Molecules, biodiversity, food and health Module 1 - Biological molecules Biological molecules Describe how hydrogen bonding occurs between water molecules, and relate this and other properties of water to the roles of water in living organisms. Describe, with the aid of diagrams, the structure of an amino acid. Structure of an Amino Acid All amino acids have the same general structure, a carboxyl group, an amino group attached to a carbon atom (-NH2) but the R group is variable, and that is the only difference between amino acids. Structure of Glycine 1
- 2. Emily Summers Describe, with the aid of diagrams, the formation and breakage of peptide bonds in the synthesis and hydrolysis of dipeptides and polypeptides. Condensation reactions make peptide bonds between amino acids. A molecule of water is released. It’s reversible, and by adding a water molecule you can break the peptide bonds. This is called hydrolysis. Explain, with the aid of diagrams, the term primary structure. Explain, with the aid of diagrams, the term secondary structure with reference to hydrogen bonding. Explain, with the aid of diagrams, the term tertiary structure, with reference to hydrophobic and hydrophilic interactions, disulphide bonds and ionic interactions Ionic Interactions weak attractions between oppositely charged parts the molecule Disulfide Bonds Two molecules of an amino acid close together, the sulphur atoms in them bond together forming this bond. (E.g. Cysteine) 2
- 3. Emily Summers Hydrophobic Water repelling groups near together in a protein they clump. Hydrophilic Water attracting groups are likely to be pushed outside, affecting the protein’s final structure. Explain, with the aid of diagrams, the term quaternary structure, with reference to the structure of haemoglobin. The quaternary structure tends to be determined by the tertiary, when it involves multiple polypeptides. E.g. Haemoglobin has a quaternary structure, it has 4 polypeptide chains. Describe, with the aid of diagrams, the structure of a collagen molecule Collagen is a strong protein that is fibrous. It is a supportive tissue in animals; it is made of three polypeptide chains that are very tightly coiled into a triple helix, interlinked by covalent bonds. Minerals are able to bind to this helix to increase rigidity. Tendons are made up mostly of collagen Walls of arteries contain collagen to prevent bursting from high pressure blood in them Cosmetic treatment for lips for a fuller appearance Compare the structure and function of haemoglobin (as an example of a globular protein) and collagen (as an example of a fibrous protein). Haemoglobin Collagen Globular protein Fibrous Protein Large variety of amino acids in it’s primary 35% of primary structure is glycine structure Has a prosthetic group- haem Doesn’t contain a prosthetic group Mostly wound into alpha helix structures Mostly left handed helix structures Describe, with the aid of diagrams, the molecular structure of alpha-glucose as an example of a monosaccharide carbohydrate. Monosaccharide carbohydrate, a hexose sugar because it has six carbon atoms in every molecule. The structure determines its solubility so it can be easily transported. It’s a source of energy for animals and plants. Its chemical bonds have lots of energy in them. 3
- 4. Emily Summers State the structural difference between alpha- and beta-glucose. Describe, with the aid of diagrams, the formation and breakage of glycosidic bonds in the synthesis and hydrolysis of a disaccharide (maltose) and a polysaccharide (amylose). Condensation- H2O removed! Hydrolysis- H2O breaks glycosidic bond. Compare and contrast the structure and functions of starch (amylose) and cellulose . Starch Cellulose Large molecules of many alpha glucose Large molecules of many beta glucose molecules joined with condensation molecules joined with condensation reactions, insoluble in water and form reactions, they are insoluble in water and granules also strong For energy storage in plants Structural found in plants where it forms cell walls 4
- 5. Emily Summers Describe, with the aid of diagrams, the structure of glycogen. Excess glucose is stored as glycogen in animals. The 1-4 and 1-6 glycosidic bonds cause branching! Meaning that the glucose can be quickly released- good for animals! Explain how the structures of glucose, starch (amylose), and glycogen and cellulose molecules relate to their functions in living organisms. Carbohydrate Example Characteristics Function in Organisms Monosaccharide Glucose (6 Carbon Small, soluble, sweet, Energy via respiration monomers sugar) crystalline Deoxyribose (5 Part of DNA Carbon sugar) information molecule Disaccharide dimers Maltose (2 glucoses) Small, soluble, sweet Sugar obtained when & crystalline starch is broken down in hydrolysis, can be split to glucose for more respiration Polysaccharide Starch & glycogen Large molecules, Energy store in plants polymers many alpha glucose as cellulose and molecules joined by glycogen in animals condensation. and fungi Insoluble in H2O and form granules Cellulose Large molecules of Structural in plants for many beta glucose cell walls. molecules joined by condensation. Insoluble in H2O and are strong. 5
- 6. Emily Summers Compare, with the aid of diagrams, the structure of a triglyceride and a phospholipid. Phospholipid Phospholipids are similar to triglycerides except that one of Triglyceride the fatty acid molecules is replaced by a phosphate group. Fatty acid tails are hydrophobic Phosphate group is hydrophilic and faces outwards. Good in the bilayer for cell membranes so water soluble substances find it hard to get through. Explain how the structures of triglyceride, phospholipid and cholesterol molecules relate to their functions in living organisms. Triglyceride molecules are used as energy storage molecules. This is good because the hydrocarbon tails of the fatty acids have lots of chemical energy that is released when broken down, so lipids contain double the energy carbohydrates do. They are insoluble because of their hydrophobic so they do not interfere with the water potential in cells that would cause water to enter cells by osmosis so they could swell/burst. Phospholipid molecules have a hydrophilic head and a hydrophobic tail. The head faces outwards and the tail faces inwards in the phospholipid bilayer on cell surface membranes, making it difficult for water soluble substances like Na+ ions and glucose to pass through. 6
- 7. Emily Summers Cholesterol is a lipid found in cell membranes for mechanical stability, and is used to make steroids. It has a hydrocarbon ring structure attached to a hydrocarbon tail. The hydrocarbon ring has a polar hydroxyl group attached to it which makes it soluble. Describe how to carry out chemical tests to identify the presence of the following molecules: protein (biuret test), reducing and non- reducing sugars (Benedict’s test), starch (iodine solution), and lipids (emulsion test). Biuret’s Test Add Benedict’s solution to the substance and heat to 80 degrees Celsius in a water bath. If the solution changes colour from blue to green-brick red then it is a reducing sugar. (Monosaccharide and disaccharide) Non reducing sugars do not react with Benedict’s solution so there would be no colour change E.g. Sucrose, formed by a condensation reaction between glucose and fructose. The formation of this bond is different to reducing sugars, so it must be boiled with hydrochloric acid, to hydrolyse/split the sucrose molecules to give glucose and fructose monosaccharides. Then add an alkali to a cool solution to neutralise it, e.g. NaCO3 solution. Then do the reducing sugar test and you should get a positive result. Starch Add iodine in a potassium iodide solution to the sample, and if there is starch the sample solution will change from yellow/brown to a dark blue/black. Negative results present no colour change. Lipids Mix the sample with ethanol, dissolving lipids present. Pour the solution into water in a separate test tube. If there is a lipid there will be a cloudy white milky emulsion near the top of the water. 7
- 8. Emily Summers Protein Add biuret reagent to a sample. The reagent contains sodium hydroxide and copper sulphate, reacting with the peptide bonds in protein turning the solution to a purple colour if there is protein, and staying blue if there is no protein. Describe how the concentration of glucose in a solution may be determined using colorimetry. A colourimeter measures the absorbance of light of a solution; the more concentrated the colour the higher the absorbance is. Make up several glucose solutions of known, different solutions Do a Benedict’s test on each solution, same amount in each case make sure there is excess reagent Remove precipitate (Centrifuge/ leave for a day) Use colourimeter to measure absorbance of Benedict’s solution remaining in each tube Record your results in a calibration curve (absorbance against glucose concentration) Test the unknown solution by using the colourimeter and reading it’s absorbance value across on the calibration graph, it will tell you the concentration. 8
- 9. Emily Summers Nucleic Acids State that deoxyribonucleic acid (DNA) is a polynucleotide, usually double-stranded, made up of nucleotides containing the bases adenine (A), thymine (T), cytosine (C) and guanine (G). A nucleotide A phosphate group Adenine and Thymine bond together with 2 hydrogen bonds, Cytosine and guanine join with 3 hydrogen bonds Deoxyribose Joined with covalent bonds (sharing of electrons) State that ribonucleic acid (RNA) is a polynucleotide, usually single-stranded, made up of nucleotides containing the bases adenine (A), uracil (U), cytosine (C) and guanine (G). 9
- 10. Emily Summers Describe, with the aid of diagrams, how hydrogen bonding between complementary base pairs (A-T, G-C) on two anti-parallel DNA polynucleotides leads to the formation of a DNA molecule, and how the twisting of DNA produces its double-helix shape. Outline, with the aid of diagrams, how DNA replicates semi-conservatively, with reference to the role of DNA polymerase. The enzyme called DNA helicase breaks the hydrogen bonds between the two polynucleotide DNA strands unzipping the helix to form two single strands, exposing the bases. Each original strand is a template for a new strand, free floating DNA nucleotides join to exposed bases on each original template strand by complementary base paring (purine pyrimidine, A- -T, G- - -C) The nucleotides on the new strand are joined by DNA polymerase, and new hydrogen bonds are formed between the bases on the old and new strand. Each DNA molecule contains one strand from the original DNA molecule and one new strand. State that a gene is a sequence of DNA nucleotides that codes for a polypeptide. Gene A gene is a length of DNA that carries the code for the synthesis of one or more specific polypeptides. 10
- 11. Emily Summers Outline the roles of DNA and RNA in living organisms (the concept of protein synthesis must be considered in outline only). The sequence of bases on DNA are code instructions for proteins, they code for the amino acid sequence present in the protein. This is a gene. There are three forms of RNA: Messenger RNA Is a strand complementary to a strand of a DNA molecule, a template strand that is a copy of the coding strand of the double helix Ribosomal RNA In ribosomes Transfer RNA Carries amino acids to the ribosomes and they are bonded together to form polypeptides. Enzymes State that enzymes are globular proteins, with a specific tertiary structure, which catalyse metabolic reactions in living organisms. Enzymes are: Globular proteins and soluble in water Able to break molecules down or build them up! Biological catalysts Specific- because they catalyse a reaction with only one type of substrate Their globular structure has a pocket called an active site Activity affected by temperature and pH (Rate of reaction) Enzymes are large molecules with hundreds of amino acids. A lot of these amino acids work to keep the specific tertiary structure of the enzyme. The function of the enzyme depends on the shape, and for the enzyme to work correctly the tertiary structure must be maintained specifically. All of the structures (primary, secondary, tertiary) of the enzyme is involved in the specific active site shape. (Where the catalytic activity of the enzyme happens) Enzymes are faster than catalysts and because they are specific to one reaction they do not produce unwanted by products. An individual cell could contain over one thousand enzymes to catalyse every process, like digestion, respiration, photosynthesis. 11
- 12. Emily Summers State that enzyme action may be intracellular or extracellular. Extracellular Enzymes catalase reactions outside of the cell Intracellular Enzymes catalase reactions inside of the cell Mould produces extracellular enzymes to digest bread. Phagocytes take in and digest bacteria using lysosomal enzymes. Describe, with the aid of diagrams, the mechanism of action of enzyme molecules, with reference to specificity, active site, lock-and- key hypothesis, induced-fit hypothesis, enzyme-substrate complex, enzyme-product complex and lowering of activation energy. Enzymes reduce the amount of activation energy needed, so reactions happen quickly at lower temperatures, because of the way the active site is shaped to fit the substrate. Enzyme’s active site is complementary to the shape of the substrate, they are specific. 12
- 13. Emily Summers Describe and explain the effects of pH, temperature, enzyme concentration and substrate concentration on enzyme activity. pH pH is the measure of the H+ ion concentration. These ions are positive so they are attracted to negatively charged ions, or parts of molecules and repelled by positive parts. Hydrogen bonds and ionic bonds hold the tertiary structure of an enzyme in place so the active site is maintained in it’s correct shape. The bonds are there because of electrostatic attraction between opposite charges on the amino acids making up the enzyme. Hydrogen ions interfere with these bonds and can alter the tertiary structure of an enzyme by altering their concentration. Enzymes have their own optimum pH, the H+ ion concentration gives the enzyme the best overall shape. Enzymes work in a narrow pH range usually, and their pH range often changes with their location. E.g. Pepsin is in the stomach and has an optimum pH of 2. Handy! Whereas Trypsin has an optimum pH of 7 which is good for the conditions of the small intestine it works in, where it digests protein. Temperature When you apply heat to molecules, they move faster in a liquid or gas and they also vibrate. The vibrations strain the bonds holding the molecules. 13
- 14. Emily Summers In large molecules like enzymes the vibration of molecules can break weaker bonds like hydrogen or ionic bonds. The weaker bonds are there in abundance in an enzyme molecule and hold the tertiary structure in place, so they maintain the active site’s correct, specific shape. Increasing temperature = Increasing bonds broken And the tertiary structure is held less in the shape of the active site needed for it to work. So rate of reaction will decrease if the substrate can’t fit in the active site. If enough of the bonds are broken, the entire tertiary structure unravels and the enzyme stops working. If the tertiary structure of an enzyme is changed enough it will not function and it is not restorable denaturation. Denaturation Changes only the tertiary structure of an enzyme so it can’t function and its function can’t be restored, which changes the active site of the enzyme. Concentration Increasing the enzyme concentration increases rate of reaction to a point, until it will not increase anymore because substrate concentration is the limiting factor. Reactions cannot be quick if there isn’t enough substrate left, and vice versa. 14
- 15. Emily Summers Describe how the effects of pH, temperature, enzyme concentration and substrate concentration on enzyme activity can be investigated experimentally. Variable Method of Keeping Reasons Constant Temperature Carrying out the enzyme Room temperature changes controlled reaction in a and fluctuations in the water bath with thermostat temperature alters the enzyme controlled reaction so results will not reflect the true action of the independent variable that is being found Enzyme Concentration Use an accurate measured Rate of reaction depends volume of enzyme-solution on concentration of enzyme molecules present; using accurate volumes of enzyme solution gives a true constant conc. Of enzyme molecules Living tissue Mass of Assume that the pieces of tissue has to be accurate tissue have the same number of enzyme molecules Whole pieces of tissue The number of enzymes same surface area and that have contact with mass substrate affects rate of reaction, e.g. surface area Substrate Concentration Accurately measured Rate of reaction depends substrate volume/mass on substrate molecule concentration pH value Use pH buffers by keeping Rate of reaction depends H+ concentration constant on pH because it alters the shape of the active site of the enzyme Explain the effects of competitive and non-competitive inhibitors on the rate of enzyme-controlled reactions, with reference to both reversible and non-reversible inhibitors. Competitive inhibitors have a similar shape to the substrate so they occupy the active site and form an enzyme inhibitor complex but no product is made. So the enzyme cannot catalyse a reaction and rate of reaction slows down. Depends on inhibitor and substrate concentration, e.g. if you increase substrate rate of reaction may increase. Non Competitive inhibitors don’t occupy the active site, but attach somewhere else on the enzyme to distort the tertiary structure of the enzyme. So the active site changes 15
- 16. Emily Summers and the substrate can’t fit anymore, so no reaction can be catalysed and reaction rate decreases. Increasing substrate concentration has no effect. Reversible inhibitors are when the inhibitor isn’t there permanently and afterwards the enzyme is unaffected. Non Reversible inhibitors are usually non competitive and the enzyme is denatured. Explain the importance of cofactors and coenzymes in enzyme-controlled reactions Coenzymes take part in the reaction and are changed, but are recycled back to take place in the next reaction. Cofactors are there to ensure an enzyme controlled reaction takes place at an appropriate rate, and some enzymes can only catalyse a reaction if a cofactor is there. State that metabolic poisons may be enzyme inhibitors, and describe the action of one named inhibitor. Lots of poisonous substances have their effects due to inhibiting or over-activating enzymes. For instance, Potassium Cyanide inhibits respiration of cells, because it is a non competitive inhibitor for a vital respiratory enzyme, cytochrome oxidase, in the mitochondria. When cytochrome oxidase is inhibited the use of oxygen is reduced and ATP cannot be produced. So the organism is only able to respire anaerobically, which builds up lactic acid in the blood increasing its acidity. State that some medicinal drugs work by inhibiting the activity of enzymes. Viral infections are treated using chemicals that act as protease inhibitors, which stop viruses from replicating by inhibiting the activity of protease- which are vital to viruses to build their new virus coats. Usually these inhibitors are competitive. Cystic Fibrosis sufferers have the problem that the passage of digestive enzymes that are usually secreted from the pancreas into the gut is blocked, leading to digestive problems. Enzymes in a tablet can overcome the problem; they are in an acid resistant coat so they’re not destructed by acid/protein digesting enzymes located in the stomach. Module 2 Food and Health Diet and Food Production Define the term “Balanced Diet” A diet that contains all the nutrients needed for health in appropriate portions 16
- 17. Emily Summers Explain how consumption of an unbalanced diet can lead to malnutrition, with reference to obesity. Malnutrition is caused by an unbalanced diet, which includes obesity- having a BMI of 30 or over, with 20% or more above the recommended weight for your height, If you consume too little or too much of each food group. BMI = mass in kg/ height in m2 Discuss the possible links between diet and coronary heart disease (CHD). There are many health risks linked to an unbalanced diet and obesity. E.g. cancer, cardiovascular disease and type two diabetes. CHD is the result of fatty depositions in the walls of the coronary arteries. (Atherosclerosis) Salt decreases blood water potential so more water is in the blood and BP +, causing hypertension, which is a too high blood pressure especially at diastolic pressure when the heart should be relaxing with a lower BP. This can damage the arteries inner lining which can help to cause atherosclerosis. Lipids Animal fat is usually saturated and plant fat is usually unsaturated. Saturated = BAD! Monounsaturated & Polyunsaturated= GOOD! Cholesterol has similar properties to triglycerides. In meat, eggs, dairy. Concentration of cholesterol in blood shouldn’t exceed 5.2 mmol dm-3 Discuss the possible effects of a high blood cholesterol level on the heart and circulatory system, with reference to high density lipoproteins (HDL) and low density lipoproteins (LDL). HDL’s are made by unsaturated fats, cholesterol and protein. They carry cholesterol from the body tissues to the liver usually. The cells in the liver have receptor sites that let the HDL’s bind onto their cell surface membranes. In the liver the cholesterol is used in cell metabolism to make bile or it can be broken down so high levels of HDL’s are linked with reducing blood cholesterol levels. This is because they decrease fatty deposition in the artery walls caused by atherosclerosis and can even help to remove depositions of it. HDL’s use unsaturated fats, which are thought to be more beneficial to health than saturated fats. LDL’s/Low density Lipoproteins are made by a combination of saturated fats, cholesterol and protein. They usually carry cholesterol from the liver to the tissues. The tissue cells contain receptor sites that let the LDL’s bind to their cell surface membranes. If there is too much saturated fat and cholesterol is consumed in the person’s diet then the blood concentration of low density lipoproteins will increase. 17
- 18. Emily Summers Different fats will affect the low density lipoprotein receptors in different ways. For example: · Saturated fats decrease LDL receptor activity · So as blood concentration of LDL increases less LDL is removed from blood and so there are higher concentrations in blood and deposits in artery walls · Polyunsaturated fats appear to increase the low density lipoprotein receptor activity so they decrease the concentration of low density lipoprotein that is present in the blood. · Additionally, monounsaturated fats appear to be able to help to remove low density lipoproteins from the blood, which is beneficial to health. Explain that humans depend on plants for food as they are the basis of all food chains . Plants are autotrophs and photosynthesise to convert light energy to glucose. They change energy from the sun to energy in a chemical form that animals can use. So we depend on plants for food. Outline how selective breeding is used to produce crop plants with high yields, disease resistance and pest resistance. Outline how selective breeding is used to produce domestic animals with high productivity. Isolation Choosing a pair of animals/plants that have the desired characteristics and allow them to reproduce Artificial Selection Offspring with the best combination of characteristics are carefully selected and allowed to reproduce Inbreeding/line breeding ^^ over many generations the characteristics are exaggerated and the breeding programmes are carefully monitored Farmers breed cattle for a high milk yield and for meat production, dairy cows can produce a massive 40l or more milk a day. Chickens are bred to produce eggs, or for meat. Egg layers can produce a huge 300 or more eggs a year, whereas normal chickens only produce 20-30 eggs per annum. Describe how the use of fertilisers and pesticides with plants and the use of antibiotics with animals can increase food production. Fertilisers replace minerals in soil like nitrates, potassium and phosphates which were removed by previous crops. They increase growth rate and size of crops produced. Pesticides kill organisms that cause disease in crops which reduce yield or kill the crop. The crops are sprayed with fungicides to reduce fungal growth in roots/leaves. Animals are treated with pesticides to kill ticks living on their skin, e.g. sheep. Infected animals can be given antibiotics to reduce spread of disease to animals farmed close to each other. Otherwise the diseases can reduce growth and reproduction. 18
- 19. Emily Summers Describe the advantages and disadvantages of using microorganisms to make food for human consumption. Advantages Disadvantages Production of protein can be faster than People don’t want to eat fungal protein or plant/animal protein food grown on waste Production can be increased/decreased Isolation of the protein is hard considering easily with demand the microorganisms are grown in big fermenters and need to be isolated from the material on which they grow No animal welfare issues The protein must be purified to ensure it isn’t contaminated Good protein source for vegetarians e.g. The conditions needed to grow these Quorn microorganisms are ideal for pathogens, which can cause infection No animal fat or cholesterol in protein The protein will not have the same taste/texture as traditional protein sources SCP production can be combined with waste removal Outline the methods that can be used to prevent food spoilage by microorganisms. Cooking Heat denatures enzymes and proteins and kills microorganisms Pasteurising Heating at 72 degrees Celsius for 15 seconds and cooling rapidly to 4 degrees Celsius to kill harmful microorganisms Drying/salting/sugar coating Dehydrates microorganisms so water leaves them by osmosis Smoking Food has a hardened and dry outer surface and the smoke has antibacterial chemicals Pickling An acid pH kills microorganisms by denaturing enzymes and proteins Irradiation Ionising radiation kills microorganisms by distorting their DNA structure Cooling/freezing Slows metabolic processes and growth, reproduction by slowing down enzyme activity. Does not kill them. Canning Food is heated and sealed in airtight cans Vacuum packing No air so microbes can’t respire aerobically 19
- 20. Emily Summers Health and disease Discuss what are meant by the terms health and disease Health is a complete state of physical, mental and social well being as well as the absence of disease or infirmity. Disease is a departure from full health caused by a malfunction of the mind or body Define and discuss the meanings of the terms parasite and pathogen. Parasite Is an organism that lives in or on another living thing causing harm to the host. External head lice Internal Tapeworm Pathogen A general term for any organism that causes disease Bacteria, fungi, viruses, protoctista Describe the causes and means of transmission of malaria, AIDS/HIV and TB. Malaria is caused by a eukaryotic organism, from the genus Plasmodium and most commonly the species Plasmodium falciparum. Malaria is spread by means of a vector. The female Anopheles mosquito carries the plasmodium from an uninfected to an infected person, they feed on blood with adapted mouthparts to penetrate a blood vessel and withdraw blood, malarial parasites live in the erythrocytes of humans and feed on Hb. HIV/AIDS The virus enters the body and is un-active, but once the virus is active and attacks/destroys T helper cells in the immune system your ability to resist infection is greatly decreased. You are open to opportunistic infections which will eventually kill the person with HIV/AIDS. Exchange of bodily fluids, e.g. blood to blood, sharing needles, unprotected sex Unscreened blood transfusions Mother to baby- across the placenta or during breast feeding TB TB is caused by a bacterium, M Bovis and Mycobacterium tuberculosis. It is usually in the lungs and although it is common it usually remains unactive or the immune system controls it, it is transmitted by a droplet infection. Overcrowding 20
- 21. Emily Summers Poor ventilation Poor health, especially with HIV/AIDS Poor diet Homelessness Contact with those who migrate from countries where TB is common Discuss the global impact of malaria, AIDS/HIV and TB. REMEMBER THE WORLD HEALTH ORGANISATION! They say good health is a human right and see that in LEDC countries there may be: Poverty Lack of shelter and pure water Poor nutrition and hygiene Insufficient health services and insufficient education of disease Malaria kills three million a year, but is limited to where the Anopheles can survive which is tropical regions like Sub Saharan Africa. Global warming is a worry. HIV/AIDS Pandemic. 45 million living with HIV/AIDS in 2005 and over half lived in sub Saharan Africa. TB Worldwide disease, new strains of Mycobacterium are resistant to drugs available to treat it. Common in sub Saharan Africa but rising in Eastern Europe. Define the terms immune response, antigen and antibody. Immune response is the specific response to a pathogen involving the action of lymphocytes and the production of antibodies. Antigens are molecules that stimulate an immune response. Antibodies are protein molecules that identify and neutralise antigens. Describe the primary defences against pathogens and parasites (including skin and mucous membranes) and outline their importance. They try to prevent pathogens from entering the body, general mechanisms. The skin Main primary defence Outer layer= epidermis Keratinocytes are made by mitosis at the base of the epidermis, during migration they dry out and the cytoplasm is replaced by keratin- called keratinisation. When these cells reach the surface they aren’t alive anymore and the dead cells come off, but this layer of dead cells are a good barrier to pathogens. 21
- 22. Emily Summers Mucous Membranes In airways, lungs and digestive system Goblet cells secrete mucus and mucus lines airway passages to trap pathogens. Cilliated epithelium beats rhythmically to waft mucus to the back of the mouth where it is swallowed down to the digestive system, the acidic stomach kills most pathogens by denaturing their enzymes Eyes are protected by tear fluid antibodies and enzymes Ear canals are lined with wax to trap pathgogens Describe, with the aid of diagrams and photographs, the structure and mode of action of phagocytes. 22
- 23. Emily Summers Describe, with the aid of diagrams, the structure of antibodies Outline the mode of action of antibodies, with reference to the neutralisation and agglutination of pathogens. Antibodies cover the pathogen binding sites and prevent the pathogen from binding to a host cell and entering the cell 23
- 24. Emily Summers A large antibody can bind lots of pathogens together so the group of pathogens are too large to enter a host cell. Compare and contrast the primary and secondary immune responses Compare and contrast active, passive, natural and artificial immunity. Active Immunity Artificial Immunity Exposure to antigen No exposure to antigen Protection development takes a longer Protection is instant period Protection lasts a long while Protection lasts a short period Memory cells made No memory cells made 24
- 25. Emily Summers Active Immunity Passive Immunity Natural Catch the disease Antibodies from mother to baby across placenta Artificial Vaccination Injected with antibodies Module 3 Biodiversity & Evolution Biodiversity Define the terms species, habitat and biodiversity A species is a group of similar individual organisms with similar appearance, biochemistry, physiology and genetics whose members are able to interbreed freely to produce fertile offspring A habitat is a place where the organism lives Biodiversity is the variety of life, the range of living organisms to be found Use Simpson’s Index (D) to calculate the biodiversity of a habitat, using the formula D = (n/N)2. D = (n/N)2. There are three species of flower in a field, red, white and blue. There are eleven organisms all together, so N = 11 There are three of the red species, five of the white and three of the blue D = 1 – ((3/11)2 + (5/11)2 + (3/11)2 = 1 – 0.36 = 0.64 Quite high! Outline the significance of both high and low values of Simpson’s Index (D). The closer to one the index is, the more diverse the habitat is. A high value indicates high biodiversity in a habit which is beneficial, a low one indicates low biodiversity in a habitat which isn’t so good, and may suggest that conservation methods might have to be put in place. Classification Define the terms classification, phylogeny and taxonomy. 25
- 26. Emily Summers Classification is arranging organisms into groups based on similarities and differences in appearance. Phylogeny is the study of the evolutionary history of groups of organisms Taxonomy is the study of classification Explain the relationship between classification and phylogeny. Describe the classification of species into the taxonomic hierarchy of domain, kingdom, phylum, class, order, family, genus and species. Outline the characteristic features of the following five kingdoms: Prokaryotae (Monera), Protoctista, Fungi, Plantae, Animalia. 26
- 27. Emily Summers Kingdom Example Features Prokaryote Bacteria Single cell, no nucleus, smaller than 5 micrometres Protoctista Algae Eukaryotic, single celled or simple multicellular Fungi Mould, yeast, mushroom Chitin cell wall, eukaryotic, saprotrophic Plantae Moss, fern, roses Eukaryotic, multicellular, cellulose cell wal, autotrophic Animalia Mammals, reptiles, birds, fish, Heterotrophic, eukaryotic, no insects cell wall, multicellular Outline the binomial system of nomenclature and the use of scientific (Latin) names for species. One international name in latin with two parts is given to every organism. The first part is the genus and is a capital letter, and the second is the species and is lower case- typed in italics or underlined when written. E.g. Homo sapien Helps to avoid confusion within scientists as they’re standard scientific names. Use a dichotomous key to identify a group of at least six plants, animals or microorganisms. 27
- 28. Emily Summers Discuss the fact that classification systems were based originally on observable features but that more recent approaches draw on a wider range of evidence to clarify relationships between organisms, including molecular evidence. Early classification systems simply used observable features to put organisms into groups, but this is problematic because the fact that some organisms look similar doesn’t mean they’re closely related, e.g. sharks and whales look similar and live in the sea, but sharks are fish and whales are mammals! Classification systems today are based on more evidence, like: Molecular evidence protein and DNA similarities, e.g. how it’s stored, closeness of bases. Anatomical Similarities in structure and function of body parts Behavioural evidence Similarities in behavior and social organization of organisms Compare and contrast the five kingdom and three domain classification systems. 28
- 29. Emily Summers Evolution Define the term variation. Variation Presence of variety of differences between individuals Discuss the fact that variation occurs within as well as between species. Describe the differences between continuous and discontinuous variation, using examples of a range of characteristics found in plants, animals and microorganisms. Continuous variation Variation in which there is a full range of intermediate phenotypes between two extremes Discontinuous variation Variation in which there are discrete groups of phenotypes with few or no individuals in between Continuous Discontinuous Height Dangling/attached ear lobes Handspan Gender Weight Blood groups Shoe Size Bacteria with absence/presence of flagella Continuous Discontinuous Affected by environment & genes Unaffected by environment, just genes Quantitative overlaps Qualitative no overlaps Controlled by a large number of genes Controlled by few/one gene (monogenic) (polygenic) No distinct categories Distinct categories Like heart rate, muscle efficiency, IQ, growth This type of variation is rare in animals rate, rate of photosynthesis but abundant in plants, like seed colour, petal colour, etc. 29
- 30. Emily Summers Explain both genetic and environmental causes of variation. Genetic Environmental Genes from our parents Linked with genetic Combination of alleles Like height is somewhat Not the same in any other living determined by your genes but thing apart from identical twins the environment plays a part on Never a complete match the height you will reach. (diet) Human cells have 25,000 Environmental changes affect different genes and a lot of them what genes in animals and plants have more than one allele, so it are activated, bringing about isn’t likely that any two changes we see individuals will have the exact Obesity diet socio-economic same allele combinations. issues. Westernized society has more overweight people than ledc’s Outline the behavioural, physiological and anatomical (structural) adaptations of organisms to their environment Behavioural Physiological/Biochemical Anatomical •Behaviour of an organism •A physiological/biochemical • Any structure that that enables it to survive it's adaptation that ensures enhances survival of the living conditions. Like when correct functioning of cell organism is an adaptation. you touch an earthworm it processes. Like yeast can Like bacteria that have quickly contracts and goes respire sigars an/aerobically flagella to allow them to back into it's burrow. This is to get energy depending on move indepedently. a behavioural adaptation the amount of O2 in the Structural adaptation. that avoids it being eaten. environment. Producing correct enzymes to respire the sugars in the environment falls under this category. 30
- 31. Emily Summers Explain the consequences of the four observations made by Darwin in proposing his theory of natural selection. Individual in a species have differences from each other – so variation is present. Offspring bare resemblance to their parents – those characteristics are inherited. There are more offspring produced than survived to maturity - they suffer from predation, disease and competition. Populations have constant sizes “Darwin concluded that individuals that were better adapted to their environment compete better than the others, survive longer and reproduce more, so passing on more of their successful characteristics to the next generation. Darwin used the memorable phrases survival of the fittest, struggle for existence and natural selection.” Biology Mad Define the term speciation. Formation of a new species from the evolution of one species geographical isolation which is an example of allopatric speciation reproductive isolation which is an example of sympatric speciation Discuss the evidence supporting the theory of evolution, with reference to fossil, DNA and molecular evidence. Outline how variation, adaptation and selection are major components of evolution. Discuss why the evolution of pesticide resistance in insects and drug resistance in microorganisms has implications for humans. Conserving biodiversity Outline the reasons for the conservation of animal and plant species, with reference to economic, ecological, ethical and aesthetic reasons. Discuss the consequences of global climate change on the biodiversity of plants and animals, with reference to changing patterns of agriculture and spread of disease. Explain the benefits for agriculture of maintaining the biodiversity of animal and plant species. Describe the conservation of endangered plant and animal species, both in situ and ex situ, with reference to the advantages and disadvantages of these two approaches. Discuss the role of botanic gardens in the ex situ conservation of rare plant species or plant species extinct in the wild, with reference to seed banks. Discuss the importance of international cooperation in species conservation with reference to the Convention in International Trade in Endangered Species (CITES) and the Rio Convention on Biodiversity. Discuss the significance of environmental impact assessments (including biodiversity estimates) for local authority planning decisions 31
- 32. Emily Summers 32